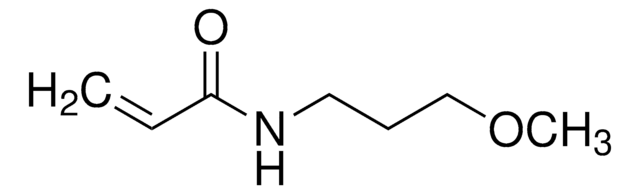
409464
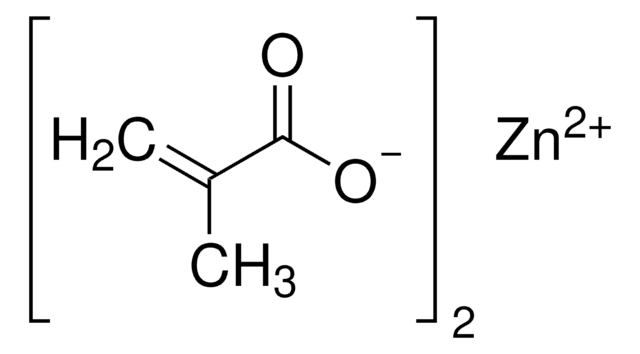
Zinc acrylate
98%
Synonyme(s) :
Acrylic acid zinc salt
About This Item
Produits recommandés
Essai
98%
Forme
powder
Pf
240-244 °C (lit.)
Chaîne SMILES
C=CC(=O)O[Zn]OC(=O)C=C
InChI
1S/2C3H4O2.Zn/c2*1-2-3(4)5;/h2*2H,1H2,(H,4,5);/q;;+2/p-2
Clé InChI
XKMZOFXGLBYJLS-UHFFFAOYSA-L
Catégories apparentées
Description générale
Application
- A monomer in the development of zinc oxide (ZnO) thin films via photopolymerization reaction. ZnO thin films exhibit favorable characteristics for use in optoelectronic devices, such as sensors, light-emitting diodes (LEDs), and photovoltaic cells. The incorporation of zinc acrylate allows for tunable properties, making these films versatile for various applications in the field of electronics and photonics.
- A key component in the formulation of gelatin/poly(zinc acrylate) hydrogel stent. Zinc acrylate incorporated into the gelatin-based hydrogel to enhance its mechanical properties contributing to its performance and suitability for use in medical applications.
- A monomer used in the synthesis of fluorescent resins through the incorporation of coumarin acid structures. The resultant fluorescent zinc acrylate resins exhibit improved antifouling properties, which are crucial for applications in marine environments.
- A monomer in the development of a poly zinc acrylate (PZA) coating that enhances the performance and stability of zinc anodes in aqueous Zn metal batteries, contributing to their high reversibility and longevity.
Mention d'avertissement
Danger
Mentions de danger
Classification des risques
Acute Tox. 3 Dermal - Acute Tox. 4 Oral - Aquatic Acute 1 - Eye Dam. 1
Code de la classe de stockage
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique