282103
Triton™ X-100 reduced
Synonyme(s) :
Polyoxyethylene (10) isooctylcyclohexyl ether
About This Item
Produits recommandés
Indice de réfraction
n20/D 1.473 (lit.)
Densité
1.029 g/mL at 25 °C (lit.)
Chaîne SMILES
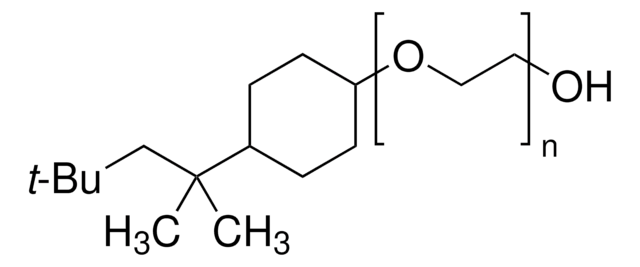
CC(C)(C)CC(C)(C)C1CCC(CC1)OCCOCCOCCOCCOCCOCCOCCO
InChI
1S/C28H56O8/c1-27(2,3)24-28(4,5)25-6-8-26(9-7-25)36-23-22-35-21-20-34-19-18-33-17-16-32-15-14-31-13-12-30-11-10-29/h25-26,29H,6-24H2,1-5H3
Clé InChI
QQJNBKDKLMCALZ-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
In biochemical and cell biology research, Triton™ X-100 is instrumental in solubilizing membrane-bound proteins and isolating lipid rafts. Its unique properties allow for the preservation of the native conformation of proteins obtained from cellular membranes in solution. Triton™ X-100 reduced is derived from the full hydrogenation of the benzene moiety of TX-100 to a cyclohexane derivative. This modified version, RTX-100, has demonstrated potential in enhancing enzyme digestion and influencing the photoisomerization of bacteriorhodopsin, showcasing its versatility and utility in advanced research applications.
Application
- as a component of LB-TT for the extraction of total protein from rat brains
- in ADP-Glo assay and Cytophos adenosine triphosphatase (ATPase) assay
- in phosphate-buffered saline (PBS) solution for the permeabilization of fibroblasts in 5′ ethynyl uridine staining, immunofluorescence, and immunolabeling
Caractéristiques et avantages
- Non-ionic surfactant
- Reduced polyoxyethylene content (~10)
- Improves solubility and dispersibility of substances
- Excellent wetting properties
- Enhances emulsification
- High purity product for research applications
Autres remarques
Informations légales
Produit comparable
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
10 - Combustible liquids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
235.4 °F - closed cup
Point d'éclair (°C)
113 °C - closed cup
Équipement de protection individuelle
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique