156329
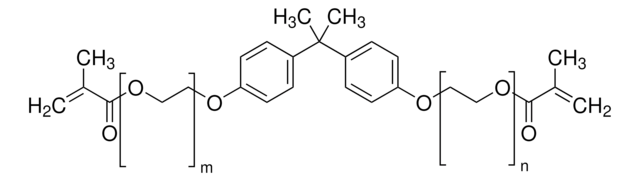
Bisphenol A dimethacrylate
>98%
Synonyme(s) :
2,2-Bis(4-hydroxyphenyl)propane dimethacrylate, 2,2-Bis(4-methacryloxyphenyl)propane, 2,2-Bis(4-methacryloyloxyphenyl)propane, 4,4′-Isopropylidenediphenol dimethacrylate, BPADMA
About This Item
Produits recommandés
Essai
>98%
Forme
solid
Pf
72-74 °C (lit.)
Température de stockage
2-8°C
Chaîne SMILES
CC(=C)C(=O)Oc1ccc(cc1)C(C)(C)c2ccc(OC(=O)C(C)=C)cc2
InChI
1S/C23H24O4/c1-15(2)21(24)26-19-11-7-17(8-12-19)23(5,6)18-9-13-20(14-10-18)27-22(25)16(3)4/h7-14H,1,3H2,2,4-6H3
Clé InChI
QUZSUMLPWDHKCJ-UHFFFAOYSA-N
Catégories apparentées
Description générale
Application
- A template in the synthesis of molecularly imprinted polymers (MIPs). The use of BPA-DMA allows for the formation of specific cavities in the polymer matrix that match the shape and functional groups of BPA, enhancing the selectivity and affinity of the resulting polymer for its target analyte.
- A co-monomer in the synthesis of photopolymerized monoliths for capillary electrochromatography.
- A key component in the production of bisphenol A-glycidyl methacrylate (Bis-GMA), which is widely used in dental restorative materials due to its mechanical properties and potential for additional functionalities like antibacterial activity.
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
dust mask type N95 (US), Eyeshields, Gloves
Faites votre choix parmi les versions les plus récentes :
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique