Recommended Products
Quality Level
Assay
≥90% (HPLC)
form
solid
mol wt
536.87 g/mol
λ
in hexane (with 2% dichloromethane)
UV absorption
λ: 458-462 nm Amax
storage temp.
−20°C
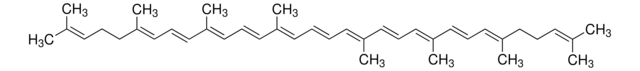
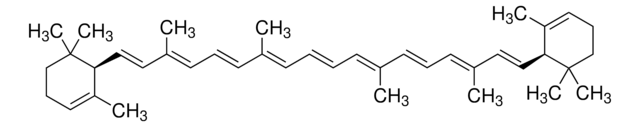
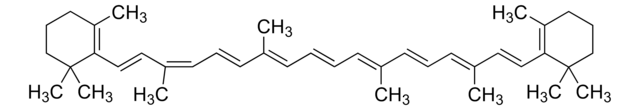
SMILES string
CC(/C=C/C1=C(C)CCCC1(C)C)=C\C=C\C(C)=C\C=C\C=C(C)\C=C\C=C(\C=C\C=C(C)\CCC=C(C)C)C
InChI
1S/C40H56/c1-32(2)18-13-21-35(5)24-15-26-36(6)25-14-22-33(3)19-11-12-20-34(4)23-16-27-37(7)29-30-39-38(8)28-17-31-40(39,9)10/h11-12,14-16,18-20,22-27,29-30H,13,17,21,28,31H2,1-10H3/b12-11+,22-14+,23-16+,26-15+,30-29+,33-19+,34-20+,35-24+,36-25+,37-27+
InChI key
HRQKOYFGHJYEFS-BXOLYSJBSA-N
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | GE17-0510-10 | Q1126 | GE17-0729-01 |
|---|---|---|---|
| particle size 45-165 μm | particle size 45-165 μm | particle size 45-165 μm (wet) | particle size 45-165 μm |
| manufacturer/tradename Cytiva 17-0510-01 | manufacturer/tradename Cytiva 17-0510-10 | manufacturer/tradename - | manufacturer/tradename Cytiva 17-0729-01 |
| packaging pack of 300 mL | packaging pack of 25 mL | packaging - | packaging pack of 300 mL |
| ligand quaternary amine | ligand quaternary amine | ligand - | ligand sulphopropyl |
| description Ion Exchanger Type (value) | description Ion Exchanger Type (value) | description - | description Ion Exchanger Type (value) |
General description
Carotenoids are tetraterpene pigments known for displaying a range of colors such as yellow, orange, red, and purple. They are mostly found in photosynthetic bacteria, certain species of archaea and fungi, algae, plants, and animals. γ-Carotene, characterized by its unsubstituted β-ionone rings, serves as a precursor to retinoids and is referred to as pro-vitamin A.[1]
Biochem/physiol Actions
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service