E50205
Ethyl 4,4,4-trifluoroacetoacetate
99%
Synonym(s):
ETFAA, Ethyl 3-oxo-4,4,4-trifluorobutyrate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
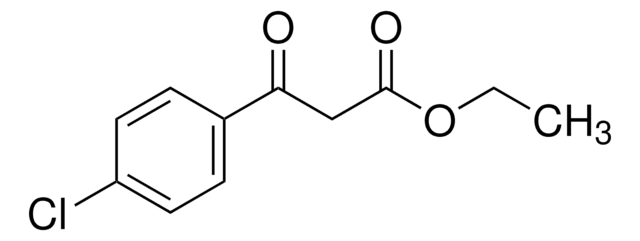
Linear Formula:
CF3COCH2CO2C2H5
CAS Number:
Molecular Weight:
184.11
Beilstein:
608353
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.375 (lit.)
bp
129-130 °C (lit.)
density
1.259 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)CC(=O)C(F)(F)F
InChI
1S/C6H7F3O3/c1-2-12-5(11)3-4(10)6(7,8)9/h2-3H2,1H3
InChI key
OCJKUQIPRNZDTK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Ethyl 4,4,4-trifluoroacetoacetate (ETFAA) is a general reagent to synthesize enantiopure trifluoromethyl-functionalized products. Applications include:
- Synthesis of (S)- and (R)-α-trifluoromethyl-aspartic acid and α- trifluoromethyl-serine from chiral CF3-oxazolidines, which is derived from ETFAA.
- Enantiopure synthesis of trifluoromethyl-β-amino acid derivatives.
- Synthesis of (2R)-2-trifluoromethyl-2-carboxyazetidine, (R)- and (S)-trifluoromethylhomoserines from oxazolidine intermediate obtained by condensing (R)-phenylglycinol with ETFAA.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
100.4 °F - closed cup
Flash Point(C)
38 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Concise synthesis of enantiopure (S)-and (R)-α-trifluoromethyl aspartic acid and α-trifluoromethyl serine from chiral trifluoromethyl oxazolidines (Fox) via a Strecker-type reaction.
Simon J, et al.
Tetrahedron Asymmetry, 22(3), 309-314 (2011)
Straightforward Synthesis of Novel Enantiopure α-Trifluoromethylated Azetidine 2-Carboxylic Acid and Homoserines.
Lensen N, et al.
Organic Letters, 17(2), 342-345 (2015)
Ethyl-4, 4, 4-trifluoroacetoacetate (ETFAA), a powerful building block for enantiopure chirons in trifluoromethyl-β-amino acid series.
Michaut V, et al.
Journal of Fluorine Chemistry, 128(8), 889-895 (2007)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service