E50000
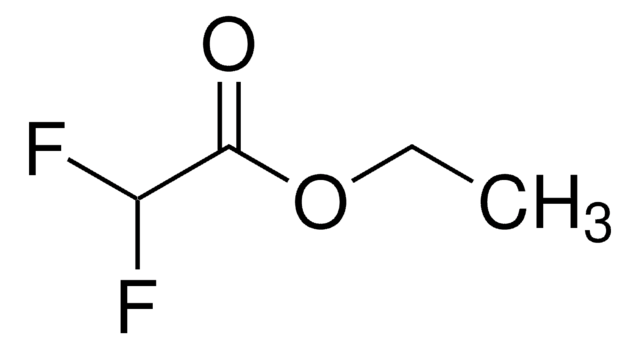
Ethyl trifluoroacetate
99%
Synonym(s):
Trifluoroacetic acid ethyl ester
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CF3COOC2H5
CAS Number:
Molecular Weight:
142.08
Beilstein:
1761411
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
refractive index
n20/D 1.307 (lit.)
bp
60-62 °C (lit.)
density
1.194 g/mL at 25 °C (lit.)
SMILES string
CCOC(=O)C(F)(F)F
InChI
1S/C4H5F3O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI key
STSCVKRWJPWALQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Ethyl trifluoroacetate can be used:
- To synthesize cyclopentenones or furans containing trifluoromethyl group from aromatic ynones.
- In the selective trifluoroacetylation of anilines catalyzed by 4-dimethylaminopyridine.
- As a starting material in the two-step electrosynthesis of trifluoroacetyltrimethylsilane (CF3COSiMe3).
- In the preparation of trifluoromethyl ketones via trifluoroacetic ester/ketone metathesis with alkyl aryl ketones.
- To synthesize o-fluorinated trifluoro acetophenones from substituted fluorobenzene.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point(F)
30.2 °F - closed cup
Flash Point(C)
-1 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Synthesis of 4-(Trifluoromethyl) cyclopentenones and 2-(Trifluoromethyl) furans by Reductive Trifluoroacetylation of Ynones
Zhang T and Maekawa H
Organic Letters, 19(24), 6602-6605 (2017)
Highly selective trifluoroacetic ester/ketone metathesis: an efficient approach to trifluoromethyl ketones and esters
Zhou Y, et al.
Tetrahedron, 70(31), 4668-4674 (2014)
Xiaoyan Li et al.
Preparative biochemistry & biotechnology, 47(9), 852-859 (2016-05-26)
Uridine 5'-diphosphate N-acetylglucosamine (UDP-GlcNAc) is a natural UDP-monosaccharide donor for bacterial glycosyltransferases, while uridine 5'-diphosphate N-trifluoacetyl glucosamine (UDP-GlcNTFA) is its synthetic mimic. The chemoenzymatic synthesis of UDP-GlcNAc and UDP-GlcNTFA was attempted by three recombinant enzymes. Recombinant N-acetylhexosamine 1-kinase was used
Qi Zhang et al.
Journal of biophotonics, 11(6), e201700339-e201700339 (2018-01-18)
Targeting cyclooxygenase-2 (COX-2) for molecular imaging is an attractive approach applicable for its overexpression in inflammation and many malignancies. Herein, for monitoring COX-2, we synthesize a specific COX-2 probe celecoxib-MPA probe (CMP), based on celecoxib and a water-soluble near-infrared dye
Heike Gerhardt et al.
Journal of chromatography. A, 1428, 280-291 (2015-06-20)
A panel of methods of general suitability for complete structural elucidation of the stereochemistry of cyclopeptides, depsipeptides and lipopeptides is presented and described in detail. The suitability of the proposed methods was exemplified on the lipopeptide poaeamide from Pseudomonas poae.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service