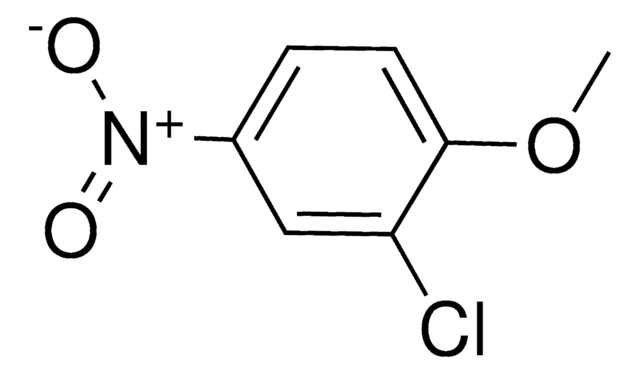
D68800
1,2-Dichloro-4-nitrobenzene
99%
Synonym(s):
3,4-Dichloronitrobenzene, asym.-Nitro-o-dichlorobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Cl2C6H3NO2
CAS Number:
Molecular Weight:
192.00
Beilstein:
1818163
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
crystals
bp
255-256 °C (lit.)
mp
39-41 °C (lit.)
SMILES string
[O-][N+](=O)c1ccc(Cl)c(Cl)c1
InChI
1S/C6H3Cl2NO2/c7-5-2-1-4(9(10)11)3-6(5)8/h1-3H
InChI key
NTBYINQTYWZXLH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
255.2 °F - closed cup
Flash Point(C)
124 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
V R Yoxall et al.
Archives of toxicology, 78(8), 477-482 (2004-03-23)
Rats were exposed to black tea (2.5% w/v) as their sole drinking liquid for either 1 day or 1 month, while controls were maintained on water. After this treatment period, all animals received a single oral dose IQ (2-amino-3-methylimidazo-[4,5-f]quinoline), and
Toshiyuki Watanabe et al.
Archives of toxicology, 78(4), 218-225 (2003-12-20)
Liver and kidney glutathione S-transferase (GST) activities to 1,2-dichloro-4-nitrobenzene (DCNB) as a substrate (GST-D activities) were measured in 280 dogs from five different breeders, and significant individual differences in this activity were observed in both organs. Interestingly, 34 out of
Shingo Arakawa et al.
Drug metabolism and disposition: the biological fate of chemicals, 38(9), 1545-1552 (2010-06-22)
A specific substrate to Mu class glutathione S-transferase (GST), 1,2-dichloro-4-nitrobenzene (DCNB), was administered to mice with a disrupted GST Mu 1 gene (Gstm1-null mice) to investigate the in vivo role of murine Gstm1 in toxicological responses to DCNB. A single
V A Catania et al.
Life sciences, 68(4), 467-474 (2001-02-24)
Gender-related differences and the regulation by testosterone of glutathione S-transferase were studied in rat jejunum. We analyzed enzyme activity and the relative content of GST subunits. Four experimental groups of adult rats were studied: normal males, castrated males, castrated males
E Egaas
Comparative biochemistry and physiology. Toxicology & pharmacology : CBP, 127(2), 117-122 (2000-11-18)
Atrazine (1,000 ppm), endosulfan (1 ppm) or butylated hydroxyanisole (BHA) (1,000 ppm) added to a semi-synthetic diet of Orthosia gothica for 2 days in the last instar did not change the soft tissue cytosolic glutathione-S-transferase (GST) activities towards 1-chloro-2,4-dinitrobenzene (CDNB)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service