D41007
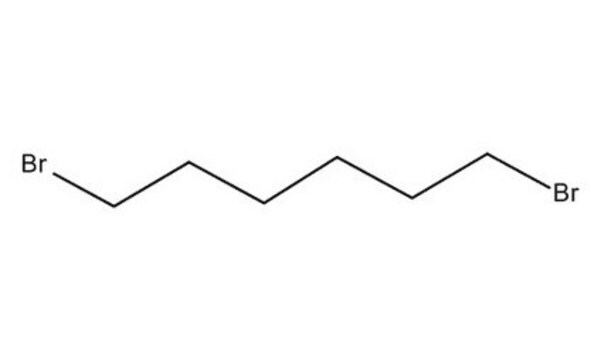
1,6-Dibromohexane
96%
Synonym(s):
Hexamethylene dibromide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Br(CH2)6Br
CAS Number:
Molecular Weight:
243.97
Beilstein:
1236322
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
liquid
refractive index
n20/D 1.507 (lit.)
bp
243 °C (lit.)
mp
−2-2.5 °C (lit.)
density
1.586 g/mL at 25 °C (lit.)
SMILES string
BrCCCCCCBr
InChI
1S/C6H12Br2/c7-5-3-1-2-4-6-8/h1-6H2
InChI key
SGRHVVLXEBNBDV-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,6-Dibromohexane is classified as an alkyl halide compound and is useful as a versatile building block in the synthesis of diverse organic compounds.
Application
1,6-Dibromohexane is generally used to introduce C6 spacer in the molecular architecture. Some of the examples are:
- Synthesis of solvent processable and conductive polyfluorene ionomers for alkaline fuel cell applications.
- Synthesis of cross-linkable regioregular poly(3-(5-hexenyl)thiophene) (P3HNT) for stabilizing the film morphology in polymer photovoltaic cells.
- Synthesis of pyrrolo-tetrathiafulvalene molecular bridge (6PTTF6) to study redox switching behavior of single molecules.
- Synthesis of water-soluble thermoresponsive polylactides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Sens. 1
Storage Class Code
10 - Combustible liquids
WGK
WGK 2
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Structure? Property Relationships in Redox-Gated Single Molecule Junctions? A Comparison of Pyrrolo-Tetrathiafulvalene and Viologen Redox Groups.
Leary E, et al.
Journal of the American Chemical Society, 130(37), 12204-12205 (2008)
Water-soluble thermoresponsive polylactides.
Jiang X, et al.
Macromolecules, 41(2), 318-324 (2008)
A soluble and conductive polyfluorene ionomer with pendant imidazolium groups for alkaline fuel cell applications.
Lin B, et al.
Macromolecules, 44(24), 9642-9649 (2011)
Nurudeen A Odewunmi et al.
ACS omega, 5(42), 27057-27071 (2020-11-03)
N 1,N 1-diallyl-N 6,N 6,N 6-tripropylhexane-1,6-diaminium chloride (NDTHDC) and its polymer poly(N 1,N 1-diallyl-N 6,N 6,N 6-tripropylhexane-1,6-diaminium chloride) (poly-NDTHDC) were synthesized and tested against API X60 carbon steel corrosion in 15 wt % HCl solution. Weight loss, electrochemical, and surface
Morphological stabilization of polymer photovoltaic cells by using cross-linkable poly (3-(5-hexenyl) thiophene).
Miyanishi S, et al.
Macromolecules, 42(5), 1610-1618 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service