All Photos(1)
About This Item
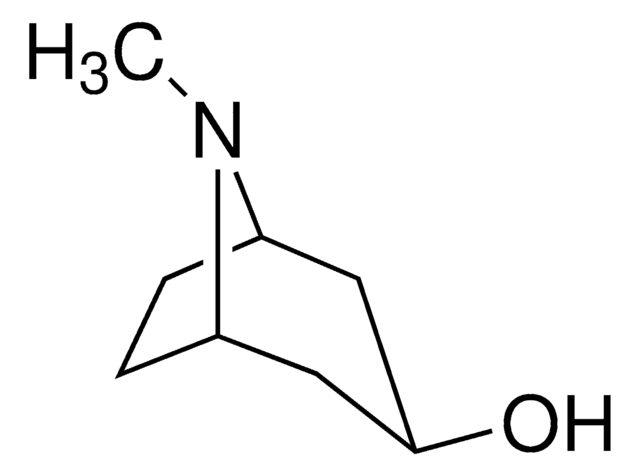
Empirical Formula (Hill Notation):
C8H15NO
CAS Number:
Molecular Weight:
141.21
Beilstein:
80188
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (NT)
form
powder
impurities
0-3% water
solubility
H2O: 0.1 g/mL, clear
functional group
hydroxyl
storage temp.
2-8°C
SMILES string
CN1[C@H]2CC[C@@H]1C[C@H](O)C2
InChI
1S/C8H15NO/c1-9-6-2-3-7(9)5-8(10)4-6/h6-8,10H,2-5H2,1H3/t6-,7+,8+
InChI key
CYHOMWAPJJPNMW-JIGDXULJSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Birgit Dräger
Phytochemistry, 67(4), 327-337 (2006-01-24)
Two stereospecific oxidoreductases constitute a branch point in tropane alkaloid metabolism. Products of tropane metabolism are the alkaloids hyoscyamine, scopolamine, cocaine, and polyhydroxylated nortropane alkaloids, the calystegines. Both tropinone reductases reduce the precursor tropinone to yield either tropine or pseudotropine.
Heike Kaiser et al.
Planta, 225(1), 127-137 (2006-07-18)
Tropinone reductases (TRs) are essential enzymes in the tropane alkaloid biosynthesis, providing either tropine for hyoscyamine and scopolamine formation or providing pseudotropine for calystegines. Two cDNAs coding for TRs were isolated from potato (Solanum tuberosum L.) tuber sprouts and expressed
Gábor Maksay et al.
Bioorganic & medicinal chemistry, 17(19), 6872-6878 (2009-09-04)
Heteroaromatic carboxylic esters of (nor)tropine were synthesized. Tropine esters displaced [(3)H]strychnine binding to glycine receptors of rat spinal cord with low Hill slopes. Two-site displacement resulted in nanomolar IC(50,1) and micromolar IC(50,2) values, and IC(50,2)/IC(50,1) ratios up to 615 depending
A Yamashita et al.
Biochemistry, 38(24), 7630-7637 (1999-07-01)
Tropinone reductase-II (TR-II) catalyzes the NADPH-dependent reduction of the carbonyl group of tropinone to a beta-hydroxyl group. The crystal structure of TR-II complexed with NADP+ and pseudotropine (psi-tropine) has been determined at 1.9 A resolution. A seven-residue peptide near the
Rawia Zayed et al.
Zeitschrift fur Naturforschung. C, Journal of biosciences, 59(11-12), 863-867 (2005-01-26)
Hairy root cultures of Brugmansia suaveolens were set up by infection of root tips with Agrobacterium rhizogenes. The successful transformation was confirmed by analysing rolC and virC genes using polymerase chain reaction (PCR). Hairy root cultures were employed to study
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service