660485
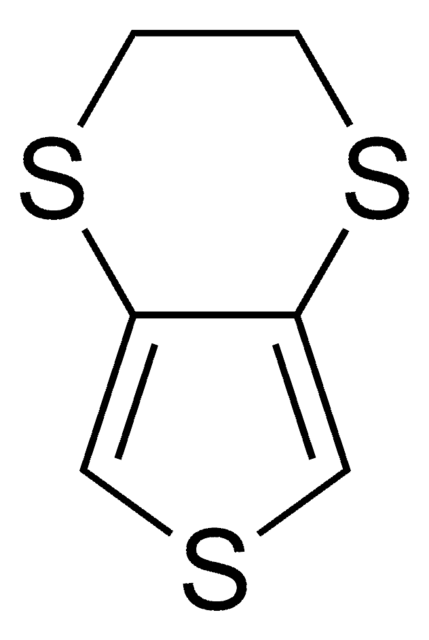
3,4-Propylenedioxythiophene
97%
Synonym(s):
3,4-Dihydro-2H-thieno[3,4-b][1,4]dioxepin, ProDOT
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H8O2S
CAS Number:
Molecular Weight:
156.20
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
solid
mp
79-83 °C
SMILES string
C1COc2cscc2OC1
InChI
1S/C7H8O2S/c1-2-8-6-4-10-5-7(6)9-3-1/h4-5H,1-3H2
InChI key
WNOOCRQGKGWSJE-UHFFFAOYSA-N
General description
3,4-Propylenedioxythiophene (PRODOT) is a conductive polymer that forms a π-conjugated system. It has good electrochromic properties with high conductivity and surface electroactivity. It forms a thin film by using layer by layer (LBL) technique and spin coating.
Application
PRODOT can be used as a conjugated polymer in the fabrication of dye sensitized solar cells (DSSCs), sensors and electrochromic materials.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A high-performance counter electrode based on poly (3, 4-alkylenedioxythiophene) for dye-sensitized solar cells
Lee K, et al.
Journal of Power Sources, 188(1), 313-318 (2009)
Triphenylamine-based dyes bearing functionalized 3, 4-propylenedioxythiophene linkers with enhanced performance for dye-sensitized solar cells
Liang Y, et al.
Organic Letters, 12(6), 1204-1207 (2010)
Jae Joon Kim et al.
Science advances, 5(3), eaaw0463-eaaw0463 (2019-03-23)
We vapor print conformal conjugated polymer electrodes directly onto living plants and use these electrodes to probe the health of actively growing specimens using bioimpedance spectroscopy. Vapor-printed polymer electrodes, unlike their adhesive thin-film counterparts, do not delaminate from microtextured living
Site-isolated electro-optic chromophores based on substituted 2, 2?-Bis (3, 4-propylenedioxythiophene) pi-conjugated bridges
Hammond SR, et al.
Chemistry of Materials, 20(10), 3425-3434 (2008)
Rational design of an electrochromic polymer with high contrast in the visible region: dibenzyl substituted poly (3, 4-propylenedioxythiophene).
Krishnamoorthy K, et al.
Journal of Materials Chemistry, 11(12), 2909-2911 (2001)
Articles
Conjugated polymers offer charge transport between inorganic, electrically conducting metals and organic, proton-conducting biological systems.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service