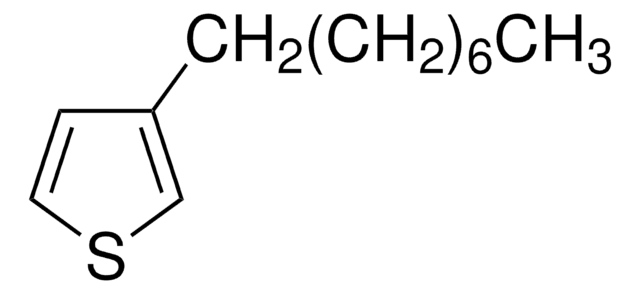399051
3-Hexylthiophene
≥99%
Synonym(s):
3-Hexylthiophene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C10H16S
CAS Number:
Molecular Weight:
168.30
Beilstein:
1617129
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
form
liquid
refractive index
n20/D 1.496 (lit.)
bp
65 °C/0.45 mmHg (lit.)
density
0.936 g/mL at 25 °C (lit.)
SMILES string
CCCCCCc1ccsc1
InChI
1S/C10H16S/c1-2-3-4-5-6-10-7-8-11-9-10/h7-9H,2-6H2,1H3
InChI key
JEDHEMYZURJGRQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
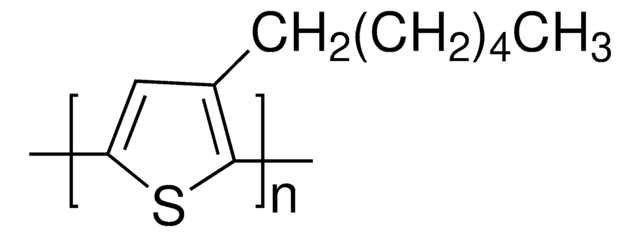
3-Hexylthiophene, a sulfur containing heterocyclic building block, is a thiophene derivative. Poly(3-hexylthiophene) (P3HT) nanofibres have been used for the preparation of organic phototransistors (OPTs).
Application
3-Hexylthiophene may be used as starting reagent in the synthesis of poly(3-hexylthiophene) (P3HT).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
X-ray and electron induced oligomerization of condensed 3-hexylthiophene.
Hernandez JE, et al.
The Journal of Physical Chemistry B, 105(35), 8339-8344 (2001)
Karolina Gebka et al.
Polymers, 11(2) (2019-04-10)
Electrochemical polymerisation is reported to be a method for readily producing copolymers of various conjugated molecules. We employed this method for mixtures of indole, carbazole or fluorene with 3-hexylthiophene (HT), in order to obtain their soluble copolymers. Although polymer films
Fangwen Zha et al.
Soft matter, 16(28), 6591-6598 (2020-07-01)
The fabrication of scaffolds with suitable chemical, physical, and electrical properties is critical for nerve cell adhesion and proliferation. Recently, electrical stimulation on conductive polymers has been applied to construct functional nerve cell scaffolds. Herein, we prepared natural polymer (cellulose)/conductive
Alexandru Oprea et al.
Analytical and bioanalytical chemistry, 412(25), 6707-6776 (2020-08-02)
Within the framework outlined in the first part of the review, the second part addresses attempts to increase receptor material performance through the use of sensor systems and chemometric methods, in conjunction with receptor preparation methods and sensor-specific tasks. Conclusions
Wouter Dierckx et al.
Nanotechnology, 26(6), 065201-065201 (2015-01-20)
Here we report the fabrication of nanofibre-based organic phototransistors (OPTs) using preformed poly(3-hexylthiophene) (P3HT) nanofibres. OPT performance is analysed based on two important parameters: photoresponsivity R and photosensitivity P. Before testing the devices as OPTs, the normal organic field-effect transistor
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service