330469
N-Phenylglycine
97%
Synonym(s):
(Phenylamino)acetic acid, Anilinoacetic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
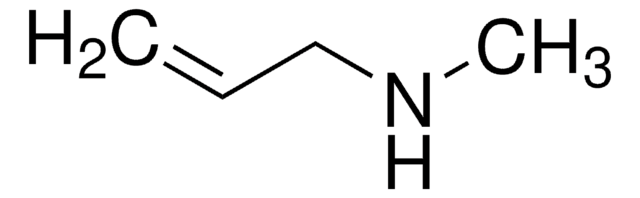
C6H5NHCH2COOH
CAS Number:
Molecular Weight:
151.16
Beilstein:
509838
EC Number:
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: solution phase peptide synthesis
mp
121-123 °C (lit.)
application(s)
peptide synthesis
SMILES string
OC(=O)CNc1ccccc1
InChI
1S/C8H9NO2/c10-8(11)6-9-7-4-2-1-3-5-7/h1-5,9H,6H2,(H,10,11)
InChI key
NPKSPKHJBVJUKB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y Imai et al.
Dental materials : official publication of the Academy of Dental Materials, 10(4), 275-277 (1994-07-01)
This research was designed to study the effect of water or carboxylic acid monomer on the polymerization of 2-hydroxyethyl methacrylate (HEMA) in order to understand the bonding mechanism of dentin bonding systems using N-phenylglycine (NPG). The polymerization of HEMA in
A D Johnston et al.
Journal of dental research, 68(9), 1337-1344 (1989-09-01)
Using bond strength measurements, we investigated a number of related compounds in order to elucidate the role of the surface-active ingredient, N-phenylglycine (NPG), in experimental two-step and three-step bonding protocols resulting in adhesive bonding to dentin. All active compounds identified
J Watkins et al.
Trends in pharmacological sciences, 15(9), 333-342 (1994-09-01)
Metabotropic glutamate receptors represent a family of G protein-coupled receptors that can be activated by L-glutamate, the principal excitatory neurotransmitter in the brain. Until recently, progress in identifying the physiological and pathological roles of metabotropic glutamate receptors has been hampered
Mohamed A Elsawy et al.
Journal of peptide science : an official publication of the European Peptide Society, 18(5), 302-311 (2012-03-28)
We have been engaged in the microwave-solid phase peptide synthesis (SPPS) synthesis of the phenylglycine (Phg)-containing pentapeptide H-Ala-Val-Pro-Phg-Tyr-NH(2) (1) previously demonstrated to bind to the so-called BIR3 domain of the anti-apoptotic protein XIAP. Analysis of the target peptide by a
P E Reifeis et al.
Operative dentistry, 20(5), 174-179 (1995-09-01)
The purpose of this study was to compare the enamel shear bond strengths achieved with four acid conditioners employed by current enamel/dentin bonding systems (maleic acid, citric acid, nitric acid, oxalic acid) with a 37% phosphoric acid etching technique. The
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service