All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H4N2S
CAS Number:
Molecular Weight:
100.14
Beilstein:
105738
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
mp
91-93 °C (lit.)
solubility
1 M HCl: soluble 50 mg/mL, clear (dark yellow-brown)
SMILES string
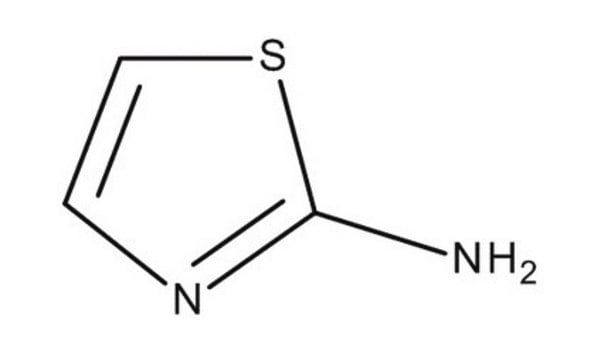
Nc1nccs1
InChI
1S/C3H4N2S/c4-3-5-1-2-6-3/h1-2H,(H2,4,5)
InChI key
RAIPHJJURHTUIC-UHFFFAOYSA-N
Application
2-Aminothiazole was used in the synthesis of 2-aminothiazole-modified silica gel. It was used in Ulmann coupling with 2-chlorobenzoic acids mediated by ultrasonic irradiation.
Biochem/physiol Actions
2-Aminothiazoles are potent cyclin-dependent kinase 5 inhibitors and are therapeutic agents for the treatment of Alzheimer′s disease and other neurodegenerative disorders.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic Communications, 37, 1853-1853 (2007)
Xin Cao et al.
Bioorganic & medicinal chemistry, 16(11), 5890-5898 (2008-05-20)
Because both c-Src and iNOS are key regulatory enzymes in tumorigenesis, a new series of 4-heteroarylamino-3-quinolinecarbonitriles as potent dual inhibitors of both enzymes were designed, prepared, and evaluated for blocking multiple signaling pathways in cancer therapy. All compounds were evaluated
David B Berry et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(44), E4160-E4169 (2013-10-17)
There is not a single pharmaceutical that halts or even slows any neurodegenerative disease. Mounting evidence shows that prions cause many neurodegenerative diseases, and arguably, scrapie and Creutzfeldt-Jakob disease prions represent the best therapeutic targets. We report here that the
Meredeth A McGowan et al.
Organic letters, 14(6), 1432-1435 (2012-03-08)
A method for the Pd-catalyzed coupling of 2-aminothiazole derivatives with aryl bromides and triflates is described. Significantly, for this class of nucleophiles, the coupling exhibits a broad substrate scope and proceeds with a reasonable catalyst loading. Furthermore, an interesting effect
Ian Bruce et al.
Bioorganic & medicinal chemistry letters, 22(17), 5445-5450 (2012-08-07)
Using a parallel synthesis approach to target a non-conserved region of the PI3K catalytic domain a pan-PI3K inhibitor 1 was elaborated to provide alpha, delta and gamma isoform selective Class I PI3K inhibitors 21, 24, 26 and 27. The compounds
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service