All Photos(1)
About This Item
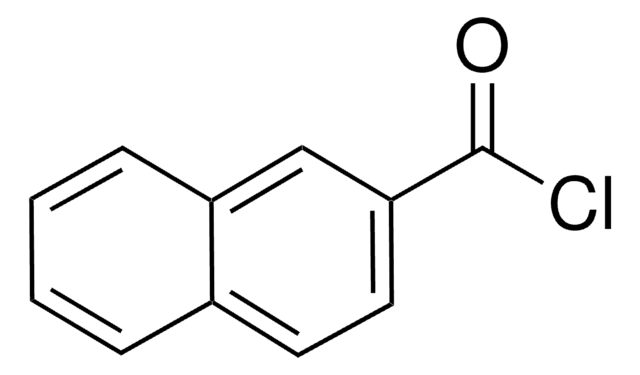
Linear Formula:
C10H7COCl
CAS Number:
Molecular Weight:
190.63
Beilstein:
775785
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
liquid
refractive index
n20/D 1.652 (lit.)
bp
190 °C/35 mmHg (lit.)
mp
16-19 °C (lit.)
density
1.265 g/mL at 25 °C (lit.)
functional group
acyl chloride
SMILES string
ClC(=O)c1cccc2ccccc12
InChI
1S/C11H7ClO/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H
InChI key
NSNPSJGHTQIXDO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Naphthoyl chloride has been used:
- as derivatization reagent in analysis of short-chained dodecyl alcohol ethoxylates and dodecyl alcohol by solid-phase extraction combined with dispersive liquid-liquid microextraction method
- as fluorescent labeling reagent in determination of T-2 and HT-2 toxins by HPLC with fluorescence detection
- in Arndt-Eistert synthesis in the presence of trimethylsilyldiazomethane
- in preparation of 2-ethyl-1-pentyl-3-(1-naphthoyl)indole
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
>230.0 °F - closed cup
Flash Point(C)
> 110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
New methods and reagents in organic synthesis. 8. Trimethylsilyldiazomethane. A new, stable, and safe reagent for the classical arndt-eistert synthesis.
Aoyama T and Shioiri T.
Tetrahedron Letters, 21(46), 4461-4462 (1980)
Vincenzo Lippolis et al.
Talanta, 74(5), 1476-1483 (2008-03-29)
T-2 and HT-2 toxins are Fusarium mycotoxins that can occur in cereals and cereal-based products. Three fluorescent labeling reagents, i.e. 1-naphthoyl chloride (1-NC), 2-naphthoyl chloride (2-NC) and pyrene-1-carbonyl cyanide (PCC), were used for the determination of T-2 and HT-2 toxins
John W Huffman et al.
Bioorganic & medicinal chemistry, 11(4), 539-549 (2003-01-23)
A series of 1-pentyl-1H-indol-3-yl-(1-naphthyl)methanes (9-11) and 2-methyl-1-pentyl-1H-indol-3-yl-(1-naphthyl)methanes (12-14) have been synthesized to investigate the hypothesis that cannabimimetic 3-(1-naphthoyl)indoles interact with the CB(1) receptor by hydrogen bonding to the carbonyl group. Indoles 9-11 have significant (K(i)=17-23nM) receptor affinity, somewhat less than
Agnieszka Zgoła-Grześkowiak et al.
Journal of chromatography. A, 1251, 40-47 (2012-07-10)
A new method was developed for preconcentration, derivatisation and analysis of short-chained dodecyl alcohol ethoxylates and dodecyl alcohol. Solid-phase extraction combined with dispersive liquid-liquid microextraction was used for preconcentration of target compounds. Several parameters were optimised including different solid phase
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service