161144
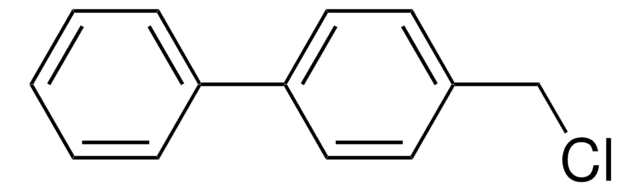
Biphenyl-4-carbonyl chloride
97%
Synonym(s):
4-Phenylbenzoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5C6H4COCl
CAS Number:
Molecular Weight:
216.66
Beilstein:
472842
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
110-112 °C (lit.)
functional group
acyl chloride
SMILES string
ClC(=O)c1ccc(cc1)-c2ccccc2
InChI
1S/C13H9ClO/c14-13(15)12-8-6-11(7-9-12)10-4-2-1-3-5-10/h1-9H
InChI key
JPVUWCPKMYXOKW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Biphenyl-4-carbonyl chloride was used in the preparation of a novel thiourea compound, N-(6-methyl pyridin-2-yl-carbamothioyl)biphenyl-4-carboxamide. It was also used in the preparation of 5-CF3-oxazole analog, 2-{4-[2-(2-biphenyl-4-yl-5-trifluoromethyl-oxazol-4-yl)-ethoxy]-phenoxy}-2-methyl-propionic acid.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Theoretical and experimental studies on N-(6-methylpyridin-2-yl-carbamothioyl) biphenyl-4-carboxamide.
Yesilkaynak T, et al.
European Journal of Chemistry, 1(1), 1-5 (2010)
Alexander G Godfrey et al.
The Journal of organic chemistry, 68(7), 2623-2632 (2003-03-29)
An improved method for the preparation of a series of oxazole-containing dual PPARalpha/gamma agonists is described. A synthetic sequence utilizing a Dakin-West reaction was devised that allows for the introduction of the oxazole ring either late in the synthetic sequence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service