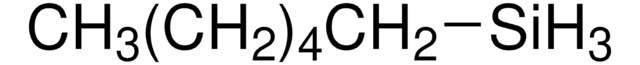148482
Diphenylsilane
97%
Synonym(s):
Diphenyl silane, Diphenylsilicon
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H5)2SiH2
CAS Number:
Molecular Weight:
184.31
Beilstein:
2935887
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.579 (lit.)
bp
95-97 °C/13 mmHg (lit.)
density
0.993 g/mL at 25 °C (lit.)
SMILES string
[SiH2](c1ccccc1)c2ccccc2
InChI
1S/C12H12Si/c1-3-7-11(8-4-1)13-12-9-5-2-6-10-12/h1-10H,13H2
InChI key
VDCSGNNYCFPWFK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Diphenylsilane is an organosilicon compound used as an amide bond coupling reagent & alkene hydrosilylation precursor.
Application
- Preparation of Bis diphenylsilane and Investigation of Its Cationic UV-Curing Material Properties: This study explores the synthesis and photosensitivity of a new material using diphenylsilane, emphasizing its potential in UV-curing applications (Y Liu et al., 2021, MDPI).
- Inkjet printing of blue phosphorescent light-emitting layer based on bis (3, 5-di (9 H-carbazol-9-yl)) diphenylsilane: Research on the use of diphenylsilane derivatives for developing blue phosphorescent materials for inkjet printing, which could be used in electronic displays (R Bail et al., 2018, RSC Advances).
accessory
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
208.4 °F - closed cup
Flash Point(C)
98 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Diphenylsilane as a coupling reagent for amide bond formation
Sayes, Morgane, et al.
Green Chemistry, 19, 5060-5064 (2017)
Saravanan Karuppiah et al.
ACS nano, 14(9), 12006-12015 (2020-09-10)
Silicon (Si) is the most promising anode candidate for the next generation of lithium-ion batteries but difficult to cycle due to its poor electronic conductivity and large volume change during cycling. Nanostructured Si-based materials allow high loading and cycling stability
Ahmad Arar et al.
Molecules (Basel, Switzerland), 25(7) (2020-04-05)
Currently, Redox Initiating Systems (RISs) of Free Radical Polymerization (FRP) are mainly based on the interaction of aromatic amines with peroxides (e.g., dibenzoyl peroxide (BPO)) that can be both toxic and unstable. In the present work, we aim to replace
Slawomir Rubinsztajn et al.
Macromolecular rapid communications, 42(5), e2000601-e2000601 (2020-12-04)
The reaction of poly(hydromethylsiloxane-co-methylphenylsiloxane) with zirconium (IV) n-propoxide in dry toluene leads to extensive scission of the siloxane chain and conversion of Si-H groups. These processes produce oligomeric siloxanes and organosilanes containing moisture sensitive propoxy and siloxy-zirconate groups. The obtained
Christopher L Rock et al.
Dalton transactions (Cambridge, England : 2003), 48(2), 461-467 (2018-11-30)
The phosphine-substituted α-diimine Ni precursor, (Ph2PPrDI)Ni, has been found to catalyze alkene hydrosilylation in the presence of Ph2SiH2 with turnover frequencies of up to 124 h-1 at 25 °C (990 h-1 at 60 °C). Moreover, the selective hydrosilylation of allylic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service