230197
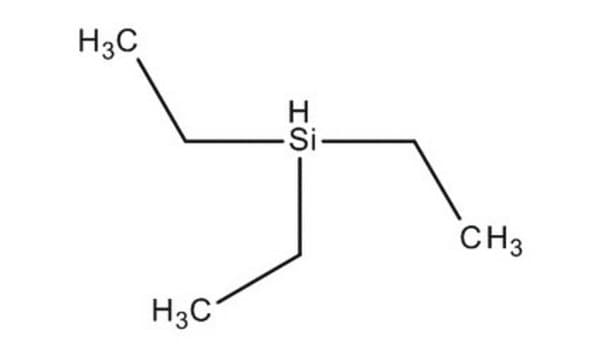
Triethylsilane
99%
Synonym(s):
NSC 93579, Triethylhydrosilane, Triethylsilicon hydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C2H5)3SiH
CAS Number:
Molecular Weight:
116.28
Beilstein:
1098278
EC Number:
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
liquid
reaction suitability
reagent type: reductant
refractive index
n20/D 1.412 (lit.)
bp
107-108 °C (lit.)
density
0.728 g/mL at 25 °C (lit.)
SMILES string
CC[SiH](CC)CC
InChI
1S/C6H16Si/c1-4-7(5-2)6-3/h7H,4-6H2,1-3H3
InChI key
AQRLNPVMDITEJU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Catalyst for:
Catalyst reactivation after catalyst polymerization of styrene
Studies involving the prediction of organosilicon flash points
- Synthesis of a spiro-oxindole blocker of Nav1.7 for the treatment of pain
- Redox initiated cationic polymerization
- Beckmann rearrangement of cyclododecanone oxime
- Regioselective reductive coupling of enones and allenes
Catalyst reactivation after catalyst polymerization of styrene
Studies involving the prediction of organosilicon flash points
Used in a study of the reduction of 2-chromanols; syn-selectivity observed with TES.
Versatile reducing agent
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
21.2 °F
Flash Point(C)
-6 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kelin Li et al.
Organic letters, 8(21), 4711-4714 (2006-10-06)
[reaction: see text] Reduction of C5-substituted 2-hydroxychromans selectively provides 2,4-cis-chromans using large silane reductants and 2,4-trans-chromans using the smaller silane PhSiH(3). The stereochemical outcome has been rationalized on the basis of a Curtin-Hammett kinetic situation arising from hydride delivery to
Zaihui Zhang et al.
Journal of medicinal chemistry, 56(2), 568-583 (2012-12-19)
Stearoyl-CoA desaturase-1 (SCD1) catalyzes de novo synthesis of monounsaturated fatty acids from saturated fatty acids. Studies have demonstrated that rodents lacking a functional SCD1 gene have an improved metabolic profile, including reduced weight gain, lower triglycerides, and improved insulin response.
Sultan Chowdhury et al.
Bioorganic & medicinal chemistry letters, 21(12), 3676-3681 (2011-05-17)
Starting from the oxindole 2a identified through a high-throughput screening campaign, a series of Na(V)1.7 blockers were developed. Following the elimination of undesirable structural features, preliminary optimization of the oxindole C-3 and N-1 substituents afforded the simplified analogue 9b, which
Emma J Ste Marie et al.
Journal of peptide science : an official publication of the European Peptide Society, 24(11), e3130-e3130 (2018-10-26)
Triisopropylsilane (TIS), a hindered hydrosilane, has long been utilized as a cation scavenger for the removal of amino acid protecting groups during peptide synthesis. However, its ability to actively remove S-protecting groups by serving as a reductant has largely been
Younggyu Kim et al.
Nucleic acids research, 36(19), 6143-6154 (2008-10-01)
We have developed an approach that enables nonradioactive, ultrasensitive (attamole sensitivity) site-specific protein-protein photocrosslinking, and we have applied the approach to the analysis of interactions of alpha-helix 2 (H2) of human TATA-element binding protein (TBP) with general transcription factor TFIIA
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service