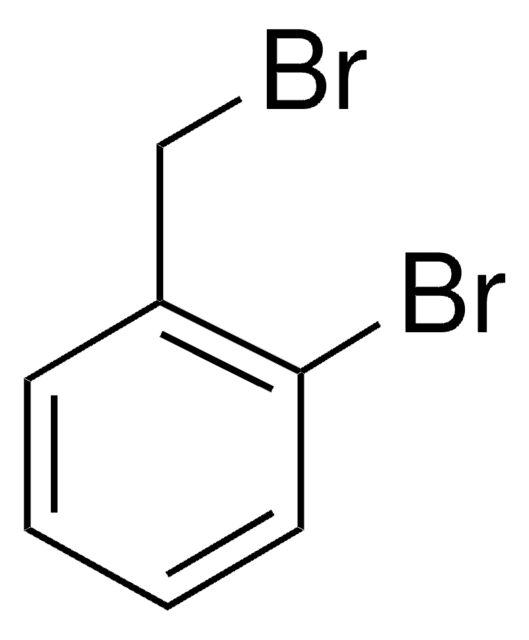
107794
2-Nitrobenzyl bromide
98%
Synonym(s):
α-Bromo-2-nitrotoluene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H4CH2Br
CAS Number:
Molecular Weight:
216.03
Beilstein:
638991
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
44-46 °C (lit.)
functional group
bromo
nitro
SMILES string
[O-][N+](=O)c1ccccc1CBr
InChI
1S/C7H6BrNO2/c8-5-6-3-1-2-4-7(6)9(10)11/h1-4H,5H2
InChI key
HXBMIQJOSHZCFX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Nitrobenzyl bromide was used for caging unprotected cysteine-containing or thiophosphorylated peptides in aqueous solution. It can be used in the synthesis of (R)- and (S)-3-amino-3,4-dihydro-1H-quinolin-2-one.
Biochem/physiol Actions
2-Nitrobenzyl bromide reacts with L-cysteine to form S-2-nitrobenzyl-cysteine which was used for modification of ultra-low-gelling-temperature (ULGT) agarose.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P Pan et al.
FEBS letters, 405(1), 81-85 (1997-03-17)
Photoreleasable molecules are important in studies of various biological phenomena, especially cell signaling. Here we report a generally applicable approach for 'caging' unprotected cysteine-containing or thiophosphorylated peptides in aqueous solution with 2-nitrobenzyl bromides. Photolysis of the caged peptides was achieved
Ying Luo et al.
Nature materials, 3(4), 249-253 (2004-03-23)
Tissue engineering aims to replace, repair or regenerate tissue/organ function, by delivering signalling molecules and cells on a three-dimensional (3D) biomaterials scaffold that supports cell infiltration and tissue organization. To control cell behaviour and ultimately induce structural and functional tissue
Kyoung-Ho Park et al.
International journal of molecular sciences, 20(16) (2019-08-21)
A kinetic study was carried out on the solvolysis of o-nitrobenzyl bromide (o-isomer, 1) and p-nitrobenzyl bromide (p-isomer, 3), and o-nitrobenzoyl chloride (o-isomer, 2) in a wide range of solvents under various temperatures. In all of the solvents without aqueous
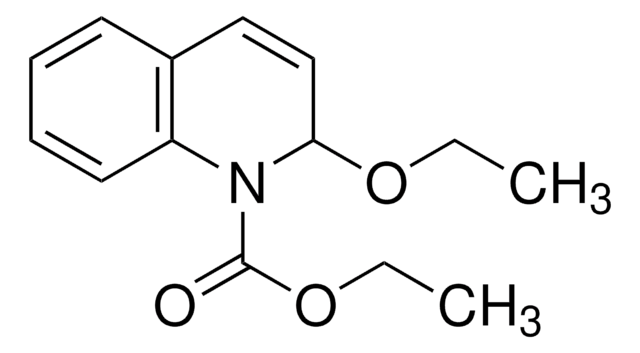
A practical synthesis of (R)-and (S)-3-amino-3, 4-dihydro-1H-quinolin-2-one.
Hulin B and Lopaze MG.
Tetrahedron Asymmetry, 15(12), 1957-1958 (2004)
Xu Yang et al.
Polymers, 12(4) (2020-04-09)
Photoresponsive polymers have attracted increasing interest for a variety of applications. Here, we report a family of photoresponsive polypeptoid-based copolymer poly(ethylene glycol)-b-poly(N-(S-(o-nitrobenzyl)-thioethyl) glycine)-co-poly(N-(2-phenylethyl) glycine) (PEG-b-PNSN-co-PNPE) synthesized by the controlled ring-opening polymerization (ROP) technique. The key feature of the design is
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service