N15553
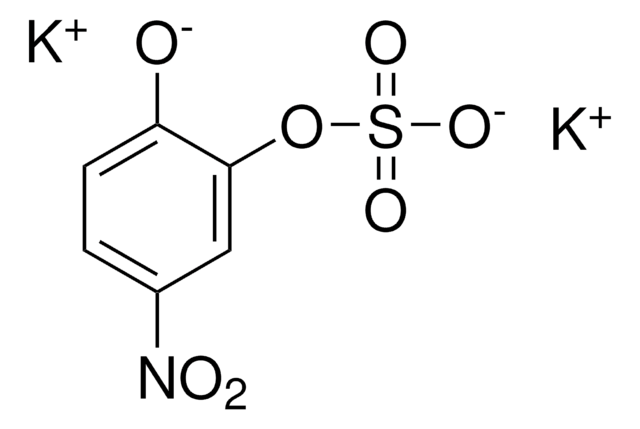
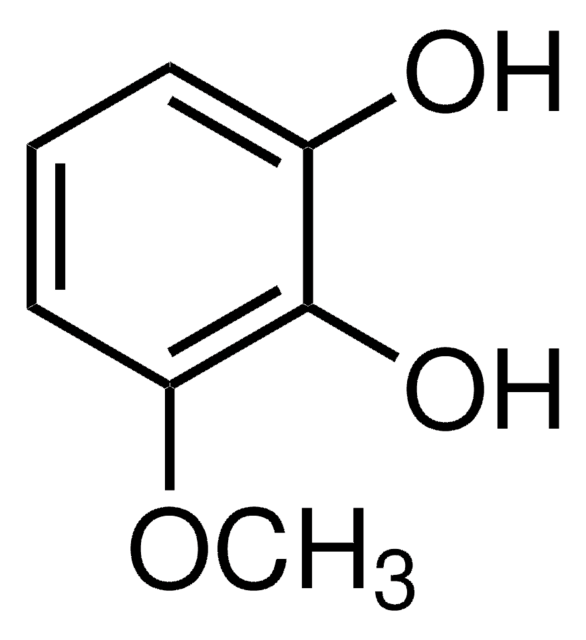
4-Nitrocatechol
≥96.0%
Synonym(s):
1,2-Dihydroxy-4-nitrobenzene, 4-Nitropyrocatechol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
O2NC6H3-1,2-(OH)2
CAS Number:
Molecular Weight:
155.11
Beilstein:
1867508
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥96.0%
form
powder
mp
173-177 °C (lit.)
SMILES string
Oc1ccc(cc1O)[N+]([O-])=O
InChI
1S/C6H5NO4/c8-5-2-1-4(7(10)11)3-6(5)9/h1-3,8-9H
InChI key
XJNPNXSISMKQEX-UHFFFAOYSA-N
Gene Information
rat ... Nos1(24598)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
W Tassaneeyakul et al.
Biochemical pharmacology, 46(11), 1975-1981 (1993-12-03)
The involvement of human cytochrome P450 (CYP) 2E1 in the hydroxylation of 4-nitrophenol (4NP) to 4-nitrocatechol (4NC) has been investigated using cDNA expression and liver microsomal kinetic and inhibitor techniques. 4NP hydroxylation by human liver microsomes and cDNA-expressed human CYP2E1
Chong Shen et al.
Chemico-biological interactions, 162(1), 53-61 (2006-06-27)
An important application of primary hepatocyte cultures is for hepatotoxicity research. In this paper, gel entrapment culture of rat hepatocytes in miniaturized BAL system were evaluated as a potential in vitro model for hepatotoxicity studies in comparison to monolayer cultures.
Archana Chauhan et al.
Environmental science & technology, 44(9), 3435-3441 (2010-04-03)
Microbial degradation studies have pointed toward the occurrence of two distinct PNP catabolic pathways in Gram positive and Gram negative bacteria. The former involves 4-nitrocatechol (4-NC), 1,2,4-benzenetriol (BT), and maleylacetate (MA) as major degradation intermediates, whereas the later proceeds via
Ewa Skrzypczak-Jankun et al.
Acta crystallographica. Section D, Biological crystallography, 60(Pt 3), 613-615 (2004-03-03)
4-Nitrocatechol (4NC) is a known inhibitor of lipoxygenase. This work presents the X-ray structure of soybean lipoxygenase-3 in complex with 4NC refined at 2.15 A resolution. The X-ray analysis shows 4NC near iron with partial occupancy, blocking access to Fe
C Shen et al.
British journal of pharmacology, 153(4), 784-791 (2007-12-12)
Rifampicin has been extensively reported to exacerbate the hepatotoxicity of isoniazid in patients with tuberculosis. However, this was controversially claimed by previous reports using rat models. This study evaluated the effect of rifampicin on isoniazid-induced hepatocyte toxicity by using human
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service