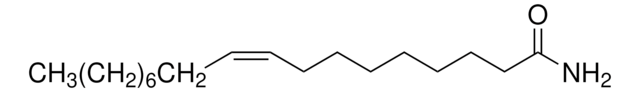
D0738000
Dextran 40 for performance test
Dextran 40 European Pharmacopoeia (EP) Reference Standard
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
41116107
NACRES:
NA.24
Recommended Products
biological source
bacterial (Leuconostoc mesenteroides)
grade
pharmaceutical primary standard
API family
dextran
analyte chemical class(es)
oligosaccharides
manufacturer/tradename
EDQM
application(s)
food and beverages
pharmaceutical (small molecule)
format
neat
storage temp.
2-8°C
General description
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
Application
Dextran 40 for performance test EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
Packaging
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
Other Notes
Sales restrictions may apply.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Jaleh Varshosaz
Expert opinion on drug delivery, 9(5), 509-523 (2012-03-22)
Dextran is a family of natural polysaccharides that is widely under investigation for use as polymeric carriers in novel drug delivery systems. The optimal drug delivery (and consequently maximum therapeutic effect) will be accomplished when carrier systems are used mainly
Mary E Pease et al.
Investigative ophthalmology & visual science, 55(4), 2564-2573 (2014-02-22)
To determine differences in scleral permeability, as measured by diffusion of macromolecules, by using fluorescence recovery after photobleaching (FRAP), with reference to differences by mouse strain, scleral region, and the effect of experimental glaucoma. In three mouse strains (B6, CD1
Assessment of glomerular size-selective function with fractional clearance of neutral dextran.
A Remuzzi et al.
The Journal of laboratory and clinical medicine, 132(5), 360-362 (1998-11-21)
J Salamon et al.
RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin, 186(4), 367-376 (2014-04-01)
The aim of this study was to establish co-labeling of mesenchymal stromal cells (MSC) for the detection of single MSC in-vivo by MRI and histological validation. Mouse MSC were co-labeled with fluorescent iron oxide micro-particles and carboxyfluorescein succinimidyl ester (CFSE).
Yingwei Wu et al.
Radiology, 271(2), 400-407 (2014-01-31)
To evaluate the feasibility of using magnetic resonance (MR) imaging and single photon emission computed tomography (SPECT)/computed tomography (CT) to visualize the in vivo recruitment of iron oxide-labeled macrophages and indium 111 ((111)In)-labeled macrophages in inflammatory bowel disease (IBD) and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service