PHR1934
Ibuprofen Impurity B
Pharmaceutical Secondary Standard; Certified Reference Material
Synonym(e):
Ibuprofen Impurity B Sodium Salt, (2RS)-2- (4-BUTYLPHENYL)PROPANOIC ACID SODIUM SALT
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Empirische Formel (Hill-System):
C13H17O2Na
CAS-Nummer:
Molekulargewicht:
228.26
UNSPSC-Code:
41116107
NACRES:
NA.24
Empfohlene Produkte
Qualität
certified reference material
pharmaceutical secondary standard
Qualitätsniveau
Agentur
traceable to Ph. Eur. B1220000
API-Familie
ibuprofen
Analysenzertifikat (CofA)
current certificate can be downloaded
Verpackung
pkg of 20 mg
Anwendung(en)
pharmaceutical (small molecule)
Format
neat
Lagertemp.
2-8°C
Allgemeine Beschreibung
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the nonsteroidal anti-inflammatory drug (NSAID)― ibuprofen, used for the treatment of mild and moderate pain such as rheumatoid arthritis, osteoarthritis, and dysmenorrhea.
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
It is an impurity of the nonsteroidal anti-inflammatory drug (NSAID)― ibuprofen, used for the treatment of mild and moderate pain such as rheumatoid arthritis, osteoarthritis, and dysmenorrhea.
Anwendung
This pharmaceutical secondary standard can also be used as follows:
- Development of a reverse-phase ultraperformance liquid chromatographic (RP-UPLC) method for the estimation of ibuprofen and diphenhydramine citrate along with their related impurities in their combined dosage form
- Simultaneous determination of ibuprofen and its 17 related impurities by an ICH validated reversed-phase high-performance liquid chromatography (RP-HPLC) method in tablets
Hinweis zur Analyse
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
Fußnote
To see an example of a Certificate of Analysis for this material enter LRAB8256 in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
Ähnliches Produkt
Produkt-Nr.
Beschreibung
Preisangaben
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Development and validation of an HPLC method for simultaneous determination of ibuprofen and 17 related compounds
Han Z, et al.
Chromatographia, 80, 1353-1360 (2017)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.








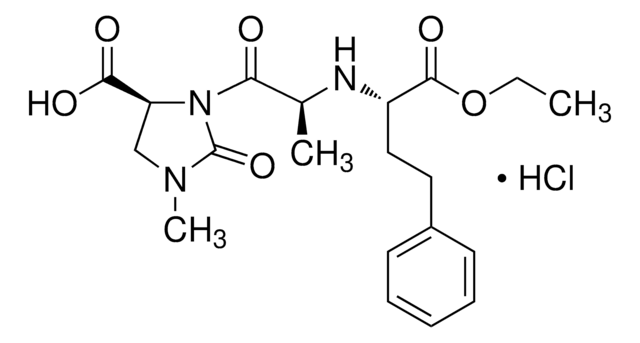
![2-[4-(1-Hydroxy-2-methylpropyl)-phenyl]-propansäure pharmaceutical impurity standard](/deepweb/assets/sigmaaldrich/product/structures/194/635/6124adcb-841c-49b5-bfa2-91cacafbf2c6/640/6124adcb-841c-49b5-bfa2-91cacafbf2c6.png)