Wichtige Dokumente
01580590
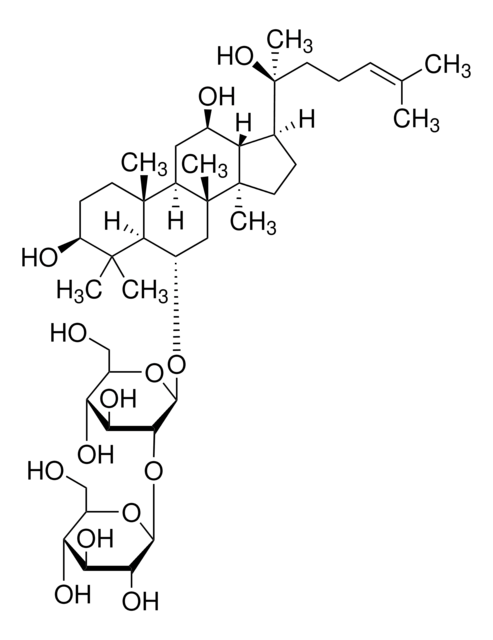
Ginsenosid Rf
primary reference standard
Synonym(e):
(3β,6α,12β)-3,12,20-Trihydroxydammar-24-en-6-yl-2-O-β-D-glucopyranosyl-β-D-glucopyranosid, Panaxosid Rf
Größe auswählen
About This Item
Empfohlene Produkte
Qualität
primary reference standard
Haltbarkeit
limited shelf life, expiry date on the label
Hersteller/Markenname
HWI
Anwendung(en)
food and beverages
Lagertemp.
−20°C
SMILES String
[H][C@]1(O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)O[C@@H]2[C@@H](O)[C@H](O)[C@@H](CO)O[C@@]2([H])O[C@H]3C[C@]4(C)[C@]([H])(C[C@@H](O)[C@]5([H])[C@]([H])(CC[C@@]45C)[C@@](C)(O)CC\C=C(/C)C)[C@@]6(C)CC[C@H](O)C(C)(C)[C@]36[H]
InChI
1S/C42H72O14/c1-20(2)10-9-13-42(8,52)21-11-15-40(6)28(21)22(45)16-26-39(5)14-12-27(46)38(3,4)35(39)23(17-41(26,40)7)53-37-34(32(50)30(48)25(19-44)55-37)56-36-33(51)31(49)29(47)24(18-43)54-36/h10,21-37,43-52H,9,11-19H2,1-8H3/t21-,22+,23-,24+,25+,26+,27-,28-,29+,30+,31-,32-,33+,34+,35-,36-,37+,39+,40+,41+,42-/m0/s1
InChIKey
UZIOUZHBUYLDHW-XUBRWZAZSA-N
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Verwandte Kategorien
Allgemeine Beschreibung
Exact content by quantitative NMR can be found on the certificate.
Anwendung
Sonstige Hinweise
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Artikel
Optimize HPLC method for ginsenoside separation using a mixture, applying it to American Ginseng root, with conditions and chromatograms shown.
In this article we present several HPTLC applications and analytical standards for ginsenosides.
Aktive Filter
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.