00878
(±)-2-Acetoxy-propionsäure
≥97.0% (GC)
Anmeldenzur Ansicht organisationsspezifischer und vertraglich vereinbarter Preise
Alle Fotos(1)
About This Item
Lineare Formel:
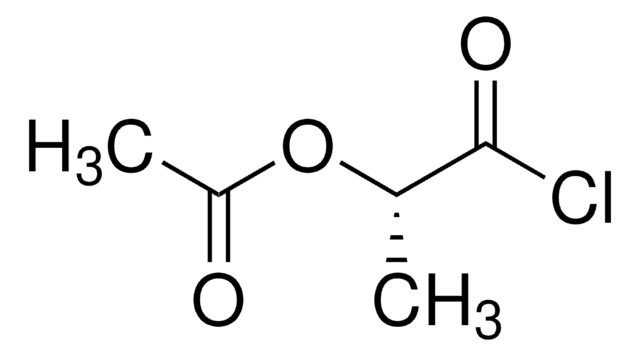
CH3COOCH(CH3)COOH
CAS-Nummer:
Molekulargewicht:
132.11
Beilstein:
1722938
MDL-Nummer:
UNSPSC-Code:
12352100
PubChem Substanz-ID:
NACRES:
NA.22
Empfohlene Produkte
Qualitätsniveau
Assay
≥97.0% (GC)
Brechungsindex
n20/D 1.423
Dichte
1.176 g/mL at 20 °C (lit.)
SMILES String
CC(OC(C)=O)C(O)=O
InChI
1S/C5H8O4/c1-3(5(7)8)9-4(2)6/h3H,1-2H3,(H,7,8)
InChIKey
WTLNOANVTIKPEE-UHFFFAOYSA-N
Verwandte Kategorien
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
10 - Combustible liquids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Choose from one of the most recent versions:
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
C T Duong et al.
Metabolic engineering, 13(6), 638-647 (2011-08-10)
Diacetyl causes an unwanted buttery off-flavor in lager beer. It is spontaneously generated from α-acetolactate, an intermediate of yeast's valine biosynthesis released during the main beer fermentation. Green lager beer has to undergo a maturation process lasting two to three
N Goupil-Feuillerat et al.
Journal of bacteriology, 179(20), 6285-6293 (1997-10-23)
The alpha-acetolactate decarboxylase gene aldB is clustered with the genes for the branched-chain amino acids (BCAA) in Lactococcus lactis subsp. lactis. It can be transcribed with BCAA genes under isoleucine regulation or independently of BCAA synthesis under the control of
H S Park et al.
Biochimica et biophysica acta, 1245(3), 366-370 (1995-12-14)
Acetolactate nonenzymatically reduced flavins, quinones and nicotinamide coenzymes in a time-dependent manner at physiological pH and moderate temperature. In the presence of excess acetolactate, the reduction of FAD and NAD+ followed pseudo-first-order kinetics. The rate of reduction was proportional to
E A Sergienko et al.
Biochemistry, 40(25), 7369-7381 (2001-06-20)
Yeast pyruvate decarboxylase (YPDC), in addition to forming its metabolic product acetaldehyde, can also carry out carboligase reactions in which the central enamine intermediate reacts with acetaldehyde or pyruvate (instead of the usual proton electrophile), resulting in the formation of
Ahmet Baykal et al.
Bioorganic chemistry, 34(6), 380-393 (2006-11-07)
In addition to the decarboxylation of 2-oxo acids, thiamin diphosphate (ThDP)-dependent decarboxylases/dehydrogenases can also carry out so-called carboligation reactions, where the central ThDP-bound enamine intermediate reacts with electrophilic substrates. For example, the enzyme yeast pyruvate decarboxylase (YPDC, from Saccharomyces cerevisiae)
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.