900692
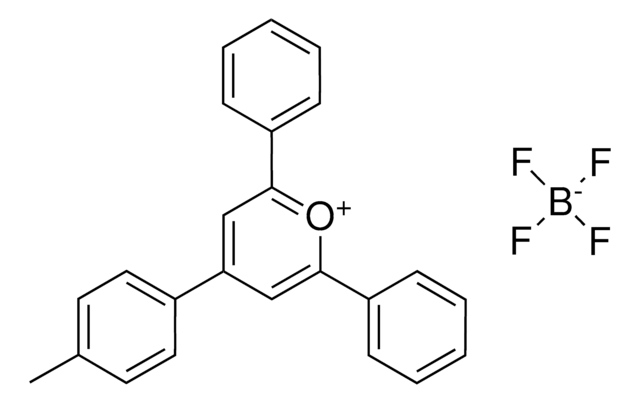
2,4,6-Tris(4-methoxyphenyl)pyrylium tetrafluoroborate
Synonym(e):
2,4,6-Tri-p-anisylpyrylium (TAP) fluoroborate
About This Item
Empfohlene Produkte
Form
powder
Qualitätsniveau
Eignung der Reaktion
reagent type: catalyst
reaction type: Photocatalysis
mp (Schmelzpunkt)
346-351 °C
SMILES String
COC(C=C1)=CC=C1C2=[O+]C(C3=CC=C(OC)C=C3)=CC(C4=CC=C(OC)C=C4)=C2.FB(F)F.[F-]
Verwandte Kategorien
Anwendung
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Sonstige Hinweise
Cyclization–endoperoxidation cascade reactions of dienes mediated by a pyrylium photoredox catalyst
Metal-Free Ring-Opening Metathesis Polymerization
Cationic Polymerization of Vinyl Ethers Controlled by Visible Light
Electron-Transfer-Induced Diels ± Alder Reactions of Indole and Exocyclic Dienes: Synthesis and Quantum-Chemical Studies
Ähnliches Produkt
Signalwort
Warning
Gefahreneinstufungen
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Verwandter Inhalt
The Nicewicz lab is focused on the discovery of new and powerful reaction methodologies that proceed via the intermediacy of highly reactive cation radical species. Included in these transformations are anti-Markovnikov selective additions of amines, alcohols, carboxylic acids, amides and mineral acids to alkenes.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.


![[Ir(dFCF3ppy)2-(5,5’-dCF3bpy)]PF6 ≥95%](/deepweb/assets/sigmaaldrich/product/structures/422/901/e00f3148-fb86-4f94-9e79-21d064c3f327/640/e00f3148-fb86-4f94-9e79-21d064c3f327.png)