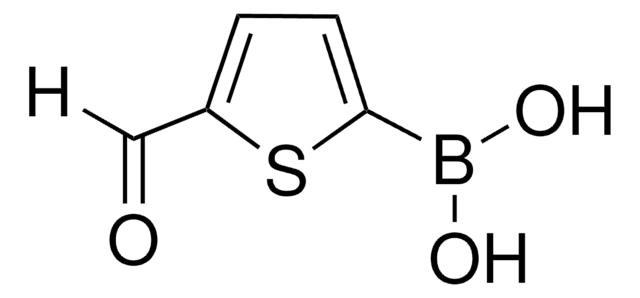
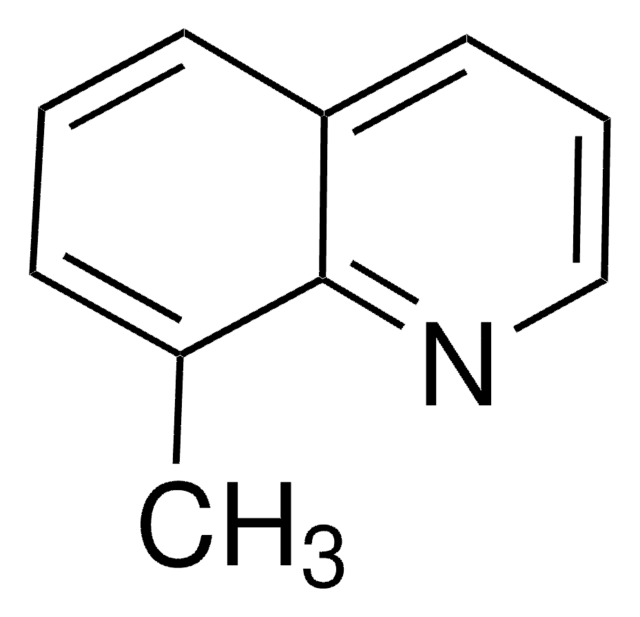
Wichtige Dokumente
900555
(N-Isocyanoimino)triphenylphosphorane
95%
Synonym(e):
Pinc
Größe auswählen
261,10 €
Listenpreis373,00 €Sparen Sie 30%Versandbereit am17. April 2025Details
Größe auswählen
About This Item
261,10 €
Listenpreis373,00 €Sparen Sie 30%Versandbereit am17. April 2025Details
Empfohlene Produkte
Qualitätsniveau
Assay
95%
Form
solid
Anwendung(en)
peptide synthesis
SMILES String
[P](=N[N+]#[C-])(c3ccccc3)(c2ccccc2)c1ccccc1
InChI
1S/C19H15N2P/c1-20-21-22(17-11-5-2-6-12-17,18-13-7-3-8-14-18)19-15-9-4-10-16-19/h2-16H
InChIKey
NIDTXBFHPXMXTR-UHFFFAOYSA-N
Verwandte Kategorien
Allgemeine Beschreibung

Anwendung
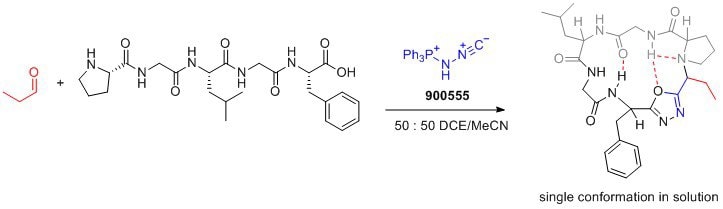
Leistungsmerkmale und Vorteile
- Bench-stable solid: Pinc is a stable reagent that can be easily handled and stored.
- Facilitates cyclization and incorporation of conformational control element: Pinc enables the formation of peptide macrocycles with a desired conformation, leading to improved drug properties.
- Amphoteric properties: Pinc′s amphoteric nature allows for the design of new multicomponent reactions, expanding its synthetic capabilities.
- Desired drug properties: The resulting peptide macrocycles exhibit enhanced membrane permeability, lipophilicity, and aqueous solubility, making them desirable for drug development.
- Versatile transformations: Pinc′s ability to form oxadiazoles opens up opportunities for the development of novel synthetic transformations.
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Zielorgane
Respiratory system
Lagerklassenschlüssel
11 - Combustible Solids
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Hier finden Sie alle aktuellen Versionen:
Analysenzertifikate (COA)
Die passende Version wird nicht angezeigt?
Wenn Sie eine bestimmte Version benötigen, können Sie anhand der Lot- oder Chargennummer nach einem spezifischen Zertifikat suchen.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Artikel
Isocyanides are widely used reagents in organic synthesis, with applications ranging from materials science to drug discovery.
Verwandter Inhalt
The Yudin laboratory is known for the development of amphoteric molecules and their application in synthesis. The corresponding reagents possess nucleophilic and electrophilic functional groups that do not prematurely react with each other.
Aktive Filter
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.
![Thieno[3,2-b]thiophene-2,5-dicarboxaldehyde 96%](/deepweb/assets/sigmaaldrich/product/structures/137/771/57dfbc98-f02d-4773-bc11-3e8b861ad74b/640/57dfbc98-f02d-4773-bc11-3e8b861ad74b.png)