682365
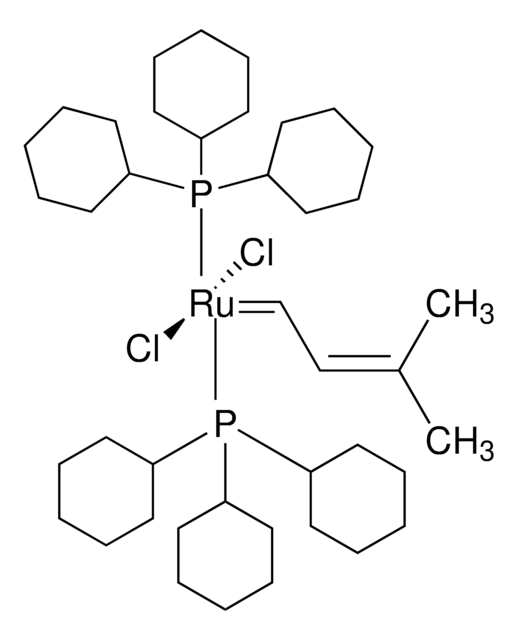
Grubbs Catalyst® M207
Umicore
Synonym(e):
[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinyliden]dichloro(3-methyl-2-butenyliden) (tricyclohexylphosphin)ruthenium(II), Isopentenyliden(1,3-dimesitylimidazolidin-2-yliden) (tricyclohexylphosphin)ruthenium(II)dichlorid, [SIMes]dichlor(3-methyl-2-butenyliden)(tricyclohexylphosphin)Ru(II)
About This Item
Empfohlene Produkte
Eignung der Reaktion
core: ruthenium
reagent type: catalyst
reaction type: Olefin Metathesis
Lagertemp.
2-8°C
SMILES String
C1CCC(CC1)P(C2CCCCC2)C3CCCCC3.C\C(C)=C/C=[Ru](Cl)(Cl)=C4N(CCN4c5c(C)cc(C)cc5C)c6c(C)cc(C)cc6C
InChI
1S/C21H26N2.C18H33P.C5H8.2ClH.Ru/c1-14-9-16(3)20(17(4)10-14)22-7-8-23(13-22)21-18(5)11-15(2)12-19(21)6;1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18;1-4-5(2)3;;;/h9-12H,7-8H2,1-6H3;16-18H,1-15H2;1,4H,2-3H3;2*1H;/q;;;;;+2/p-2
InChIKey
LCOFYVWULBZOTA-UHFFFAOYSA-L
Suchen Sie nach ähnlichen Produkten? Aufrufen Leitfaden zum Produktvergleich
Allgemeine Beschreibung
Anwendung
Sonstige Hinweise
Rechtliche Hinweise
Signalwort
Warning
H-Sätze
Gefahreneinstufungen
Flam. Sol. 2
Lagerklassenschlüssel
4.1B - Flammable solid hazardous materials
WGK
WGK 3
Flammpunkt (°F)
Not applicable
Flammpunkt (°C)
Not applicable
Persönliche Schutzausrüstung
Eyeshields, Gloves, type N95 (US)
Analysenzertifikate (COA)
Suchen Sie nach Analysenzertifikate (COA), indem Sie die Lot-/Chargennummer des Produkts eingeben. Lot- und Chargennummern sind auf dem Produktetikett hinter den Wörtern ‘Lot’ oder ‘Batch’ (Lot oder Charge) zu finden.
Besitzen Sie dieses Produkt bereits?
In der Dokumentenbibliothek finden Sie die Dokumentation zu den Produkten, die Sie kürzlich erworben haben.
Kunden haben sich ebenfalls angesehen
Verwandter Inhalt
Research in the Grubbs group has centered on the development and application of a suite of highly active, selective, and bench stable ruthenium alkylidene complexes capable of catalyzing versatile olefin metatheses.
Unser Team von Wissenschaftlern verfügt über Erfahrung in allen Forschungsbereichen einschließlich Life Science, Materialwissenschaften, chemischer Synthese, Chromatographie, Analytik und vielen mehr..
Setzen Sie sich mit dem technischen Dienst in Verbindung.



![[1,3-Bis-(2,4,6-trimethylphenyl)-2-imidazolidinyliden]-dichloro-(benzyliden)-bis-(3-brompyridin)-ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/261/898/e64eea4e-5a09-4c7d-b400-c43b3517de2a/640/e64eea4e-5a09-4c7d-b400-c43b3517de2a.png)

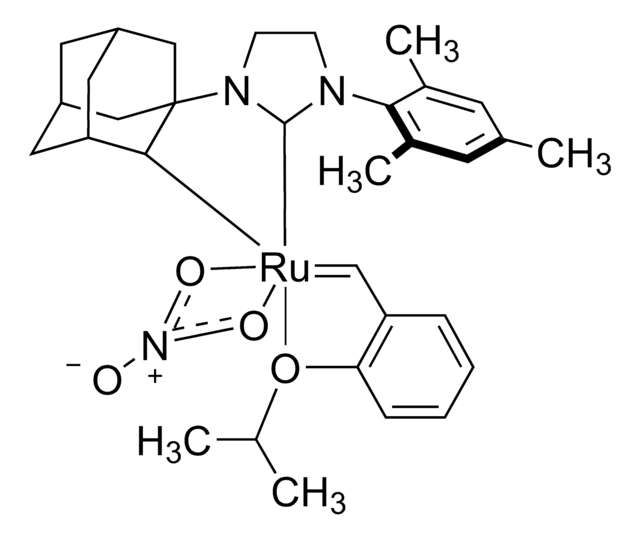
![[1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinyliden]dichloro[3-(2-pyridinyl-κN)propyliden-κC]ruthenium(II)](/deepweb/assets/sigmaaldrich/product/structures/416/586/0907c050-f5d7-4a53-b22f-23170c2703c2/640/0907c050-f5d7-4a53-b22f-23170c2703c2.png)

![Dichloro-[1,3-bis-(2,6-isopropylphenyl)-2-imidazolidinyliden]-(2-isopropoxyphenylmethylen)-ruthenium(II) Umicore](/deepweb/assets/sigmaaldrich/product/structures/221/319/afb08bfc-f0e6-419f-a7b7-cb11fa98e87f/640/afb08bfc-f0e6-419f-a7b7-cb11fa98e87f.png)