SML0761
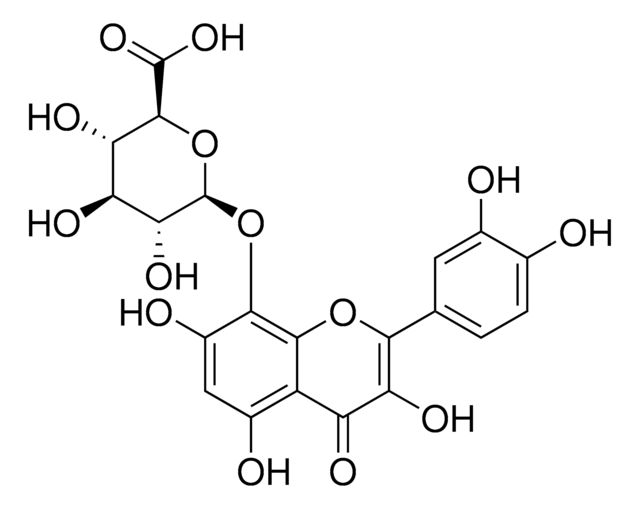
Gossypin
≥90% (HPLC)
Synonym(s):
2-(3,4-Dihydroxyphenyl)-8-(β-D-glucopyranosyloxy)-3,5,7-trihydroxy- 4H-1-benzopyran-4-one, 3,3′,4′,5,7,8-Hexahydroxyflavone 8-glucoside, Gossypetin 8-glucoside
About This Item
Recommended Products
biological source
Hibiscus vitifolius
Assay
≥90% (HPLC)
form
powder
storage condition
desiccated
color
, light yellow to dark green-yellow
solubility
DMSO: 15 mg/mL, clear
storage temp.
−20°C
SMILES string
OC[C@H]1O[C@@H](Oc2c(O)cc(O)c3C(=O)C(O)=C(Oc23)c4ccc(O)c(O)c4)[C@H](O)[C@@H](O)[C@@H]1O
InChI
1S/C21H20O13/c22-5-11-13(27)15(29)17(31)21(32-11)34-19-10(26)4-9(25)12-14(28)16(30)18(33-20(12)19)6-1-2-7(23)8(24)3-6/h1-4,11,13,15,17,21-27,29-31H,5H2/t11-,13-,15+,17-,21+/m1/s1
InChI key
SJRXVLUZMMDCNG-KKPQBLLMSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The C-terminal c-Src kinase (Csk) is a 50-kDa cytosolic tyrosine kinase expressed in all examined cell types
Review properties, activators and inhibitors, and available products for researching cyclin-dependent kinases (CDKs).
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service