All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C14H13NO
CAS Number:
Molecular Weight:
211.26
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
mp
78-82 °C (lit.)
SMILES string
OCCn1c2ccccc2c3ccccc13
InChI
1S/C14H13NO/c16-10-9-15-13-7-3-1-5-11(13)12-6-2-4-8-14(12)15/h1-8,16H,9-10H2
InChI key
IIKVAWLYHHZRGV-UHFFFAOYSA-N
Related Categories
General description
9H-Carbazole-9-ethanol (ECOH) is a carbazole derivative.
Application
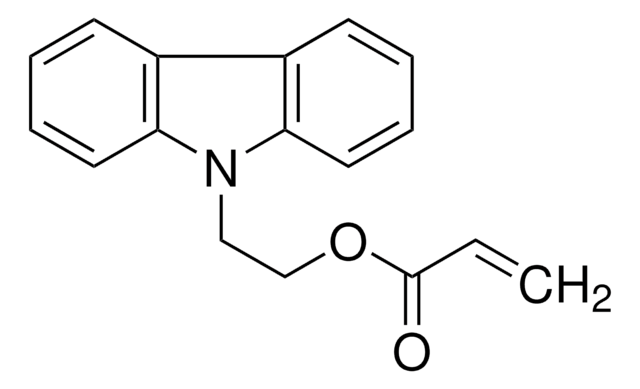
9H-Carbazole-9-ethanol may be used to synthesize 2-(9H-carbazol-9-yl)ethyl methacrylate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Thermal aging of single-layer polymer light-emitting diodes composed of a carbazole and oxadiazole containing copolymer doped with singlet or triplet emitters.
Rungta P, et al.
Synthetic Metals, 160(23), 2486-2493 (2010)
Rukiye Ayranci et al.
The Analyst, 142(18), 3407-3415 (2017-08-22)
Herein, we report the synthesis and characterization of a new rhodamine-based monomer (RD-CZ), and an investigation of the optical and electrochemical properties of the corresponding polymer (P(RD-CZ)), which was electropolymerized on an ITO electrode. The resulting P(RD-CZ) polymer film was
Yifen Lin et al.
Food chemistry, 264, 1-8 (2018-06-02)
Disassembly of cell wall polysaccharides accompanied with softening is very common in harvested fruits. To develop a facile postharvest approach, which can be used at ambient temperature, for suppressing softening and maintaining higher nutritive cell wall polysaccharides of Younai plums
Synthesis and photophysical properties of a poly (methyl methacrylate) polymer with carbazolyl side groups.
Martins TD, et al.
Journal of the Brazilian Chemical Society, 19(8), 1450-1461 (2008)
Poly(methyl methacrylate)copolymers containing pendant carbazole and oxadiazole moieties for applications in single-layer organic light emitting devices.
Evanoff DD, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 46(23), 7882-7897 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service