A-903
Alprazolam solution
1.0 mg/mL in methanol, ampule of 1 mL, certified reference material, Cerilliant®
About This Item
Recommended Products
grade
certified reference material
Quality Level
form
liquid
feature
SNAP-N-SPIKE®, SNAP-N-SHOOT®
packaging
ampule of 1 mL
manufacturer/tradename
Cerilliant®
drug control
Narcotic Licence Schedule B (Switzerland); psicótropo (Spain); Decreto Lei 15/93: Tabela IV (Portugal)
concentration
1.0 mg/mL in methanol
technique(s)
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
application(s)
clinical testing
format
single component solution
storage temp.
−20°C
SMILES string
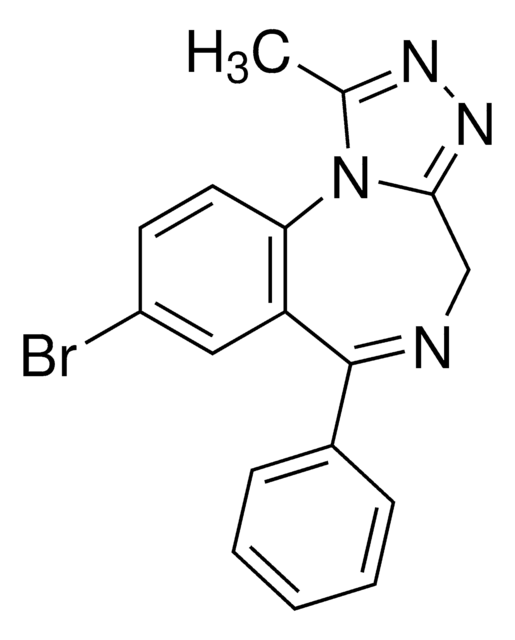
Cc1nnc2CN=C(c3ccccc3)c4cc(Cl)ccc4-n12
InChI
1S/C17H13ClN4/c1-11-20-21-16-10-19-17(12-5-3-2-4-6-12)14-9-13(18)7-8-15(14)22(11)16/h2-9H,10H2,1H3
InChI key
VREFGVBLTWBCJP-UHFFFAOYSA-N
Gene Information
human ... GABRA1(2554) , GABRA2(2555) , GABRA3(2556) , GABRA4(2557) , GABRA5(2558) , GABRA6(2559) , GABRB1(2560) , GABRB2(2561) , GABRB3(2562) , GABRD(2563) , GABRE(2564) , GABRG1(2565) , GABRG2(2566) , GABRG3(2567) , GABRP(2568) , GABRQ(55879)
Looking for similar products? Visit Product Comparison Guide
General description
Application
- Alprazolam Solid Lipid Nanoparticles for Sustained Release: This study explores the development of Compritol-based Alprazolam solid lipid nanoparticles, demonstrating a novel approach for sustained release formulations in benzodiazepine biochemistry. The technique is critical for improving the pharmacokinetics of anxiolytic compounds, ensuring controlled release and enhanced bioavailability (Rao et al., 2022).
- Extraction of Alprazolam in Biological Samples: Application of dispersive solid-phase extraction using nanographene oxide grafted with α-pyridylamine for alprazolam, which is significant for forensic and clinical toxicology. This method enhances the precision of benzodiazepine detection in complex biological matrices, pivotal for neuropharmacological studies (Parsayi Arvand et al., 2023).
- Raman Spectroscopy in Drug Abuse Analysis: Utilization of portable Raman spectroscopy for the detection of drugs of abuse, including Alprazolam. This research underscores the importance of Alprazolam research solutions in developing rapid and non-destructive testing methods, contributing to advancements in forensic science (Santos et al., 2022).
Legal Information
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
Target Organs
Eyes,Central nervous system
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
49.5 °F - closed cup
Flash Point(C)
9.7 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service