M13203
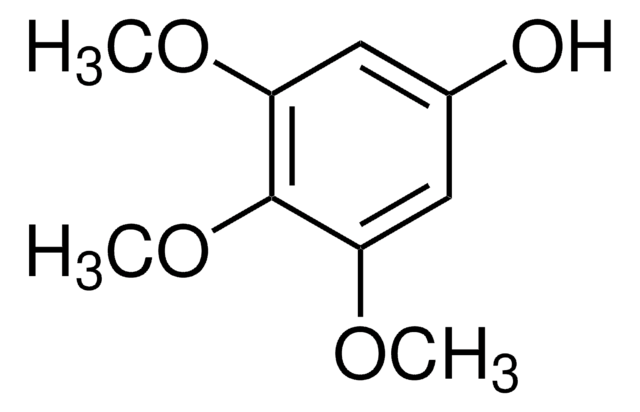
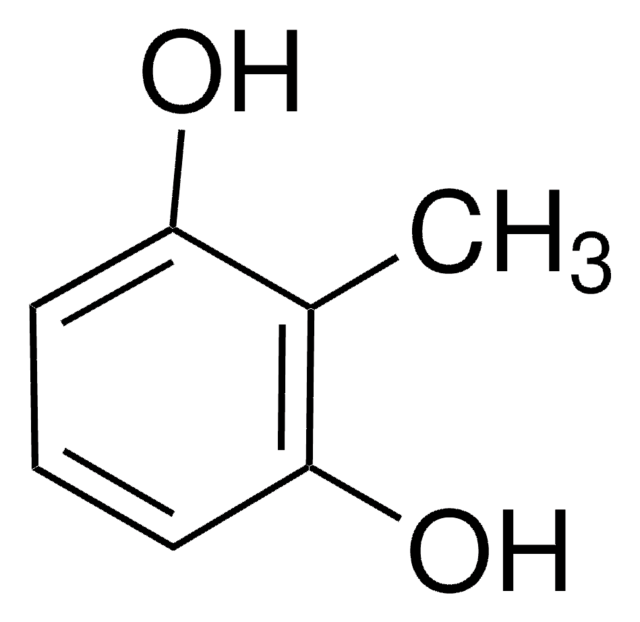
3-Methoxycatechol
99%
Synonym(s):
1,2-Dihydroxy-3-methoxybenzene, 3-Methoxypyrocatechol, Pyrogallol monomethyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3OC6H3(OH)2
CAS Number:
Molecular Weight:
140.14
Beilstein:
1909165
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
bp
146-147 °C/15 mmHg (lit.)
mp
38-43 °C (lit.)
SMILES string
COc1cccc(O)c1O
InChI
1S/C7H8O3/c1-10-6-4-2-3-5(8)7(6)9/h2-4,8-9H,1H3
InChI key
LPYUENQFPVNPHY-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Hirose et al.
Japanese journal of cancer research : Gann, 81(9), 857-861 (1990-09-01)
The effects of sodium nitrite (NaNO2) and catechol or 3-methoxycatechol in combination were examined in male F344 rats. Animals were treated with 0.3% NaNO2 in the drinking water and 0.8% catechol or 2% 3-methoxycatechol in powdered diet for 24 weeks.
M Kawabe et al.
Japanese journal of cancer research : Gann, 85(1), 17-25 (1994-01-01)
The effects of combined treatment with NaNO2 and phenolic compounds on N-methyl-N'-nitro-N-nitrosoguanidine (MNNG) stomach carcinogenesis were investigated in F344 rats. In the first experiment, groups of 15-20 male rats were treated with an intragastric dose of 150 mg/kg body weight
Keith M Anderson et al.
The Journal of organic chemistry, 72(26), 9875-9880 (2007-11-30)
A novel universal support for deoxyribo- and ribonucleic acid synthesis has been developed. The support, constructed from 1,4-dimethoxycatechol, represents an improvement over existing universal supports because of its ability to cleave and deprotect under mild conditions in standard reagents. Because
M Hirose et al.
Cancer research, 53(1), 32-37 (1993-01-01)
Effects of simultaneous treatment with NaNO2 and butylated hydroxyanisole, catechol, or 3-methoxycatechol were examined in a rat multiorgan carcinogenesis model. Groups of 15 animals were given a single i.p. injection of 100 mg/kg of body weight diethylnitrosamine, 4 i.p. injections
Ying Tao et al.
Journal of bacteriology, 186(14), 4705-4713 (2004-07-03)
Wild-type toluene 4-monooxygenase (T4MO) of Pseudomonas mendocina KR1 oxidizes toluene to p-cresol (96%) and oxidizes benzene sequentially to phenol, to catechol, and to 1,2,3-trihydroxybenzene. In this study T4MO was found to oxidize o-cresol to 3-methylcatechol (91%) and methylhydroquinone (9%), to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service