B56404
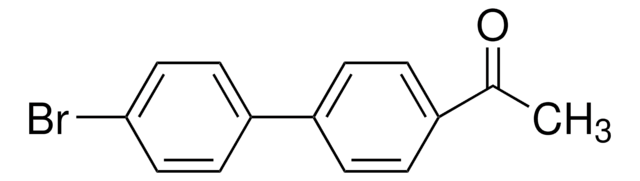
4′-Bromoacetophenone
98%
Synonym(s):
1-Acetyl-4-bromobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
BrC6H4COCH3
CAS Number:
Molecular Weight:
199.04
Beilstein:
386015
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
bp
255 °C (lit.)
mp
49-51 °C (lit.)
SMILES string
CC(=O)c1ccc(Br)cc1
InChI
1S/C8H7BrO/c1-6(10)7-2-4-8(9)5-3-7/h2-5H,1H3
InChI key
WYECURVXVYPVAT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
4′-Bromoacetophenone is used as an organic building block in Heck and Suzuki coupling reactions. Generates phenyl radicals upon UV irradiation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
>212.0 °F
Flash Point(C)
> 100 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
In vitro histamine release from basophils of asthmatic and atopic individuals in D2O.
R Tung et al.
Journal of immunology (Baltimore, Md. : 1950), 128(5), 2067-2072 (1982-05-01)
Mechanochemical solid-state Suzuki reactions using an in situ generated base.
Franziska Schneider et al.
ChemSusChem, 1(7), 622-625 (2008-08-15)
Gagan Chouhan et al.
Chemical communications (Cambridge, England), (45)(45), 4809-4811 (2007-11-16)
Magnetic nanoparticle-supported proline ligand was prepared and used for the CuI catalyzed Ullmann-type coupling reactions of aryl/heteroaryl bromides with various nitrogen heterocycles to form the corresponding N-aryl products in good to excellent yields; furthermore, this magnetic nanoparticle-supported proline ligand could
R Jeon et al.
Archives of pharmacal research, 24(1), 39-43 (2001-03-10)
4'-Bromoacetophenone analogs, which are able to generate monophenyl radicals capable of hydrogen atom abstraction, were investigated as possible photoinducible DNA cleaving agents. The potential of 4'-bromoacetophenone as a possible new DNA cleaver is explored. Pyrrolecarboxatmid conjugated 4'-bromoacetophenones, in particular, DNA
Lidiane S Araújo et al.
Marine drugs, 9(5), 889-905 (2011-06-16)
Several microorganisms were isolated from soil/sediment samples of Antarctic Peninsula. The enrichment technique using (RS)-1-(phenyl)ethanol as a carbon source allowed us to isolate 232 psychrophile/psychrotroph microorganisms. We also evaluated the enzyme activity (oxidoreductases) for enantioselective oxidation reactions, by using derivatives
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service