B4856
Diphenylmethanol
99%
Synonym(s):
Benzhydrol, Benzhydryl alcohol, Diphenyl carbinol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(C6H5)2CHOH
CAS Number:
Molecular Weight:
184.23
Beilstein:
1424379
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
bp
297-298 °C (lit.)
mp
65-67 °C (lit.)
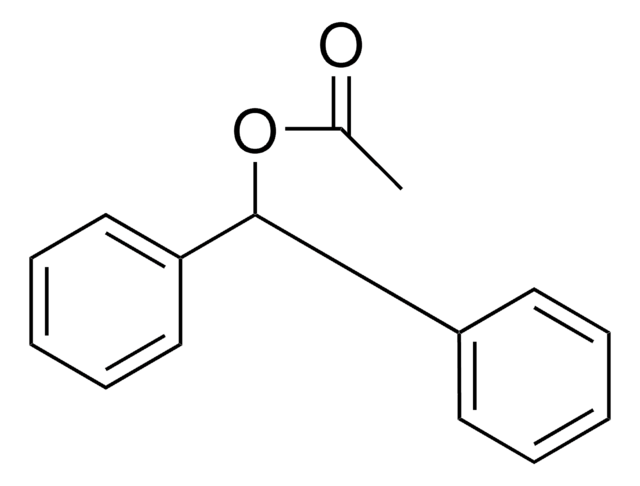
SMILES string
OC(c1ccccc1)c2ccccc2
InChI
1S/C13H12O/c14-13(11-7-3-1-4-8-11)12-9-5-2-6-10-12/h1-10,13-14H
InChI key
QILSFLSDHQAZET-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Diphenylmethanol is used in the carbene-catalyzed dynamic kinetic resolution of α,α-disubstituted carboxylic esters. It can also be used in the chiral resolution of free-base and Ni(II) porphyrinoid complexes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Helimeric porphyrinoids: stereostructure and chiral resolution of meso-tetraarylmorpholinochlorins.
Bru?ckner C, et al.
Journal of the American Chemical Society, 133(22), 8740-8752 (2011)
Carbene-Catalyzed Dynamic Kinetic Resolution of Carboxylic Esters.
Chen X, et al.
Journal of the American Chemical Society, 138(23), 7212-7215 (2016)
Masanori Ichikawa et al.
Bioorganic & medicinal chemistry, 19(6), 1930-1949 (2011-03-01)
To obtain small and efficient squalene synthase inhibitors, a flexible 2-aminobenzhydrol open form structure was designed and showed potent inhibitory activity comparable to 4,1-benzoxazepin compounds. Further chemical modification led to the discovery of a novel template with a strong squalene
Kinetics of the hydrolysis of drugs containing the diphenyl-methyl-ether group.
O O Komolafe
Materia medica Polona. Polish journal of medicine and pharmacy, 17(2), 98-102 (1985-04-01)
Masanori Ichikawa et al.
Bioorganic & medicinal chemistry, 19(17), 5207-5224 (2011-08-02)
We have recently reported the discovery of the new benzhydrol template, which has a highly potent inhibitory activity for squalene synthase, as typified by compound 1 (SSI IC(50)=0.85 nM). However, it was composed of a pair of easy rotatable atropisomers.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service