A56655
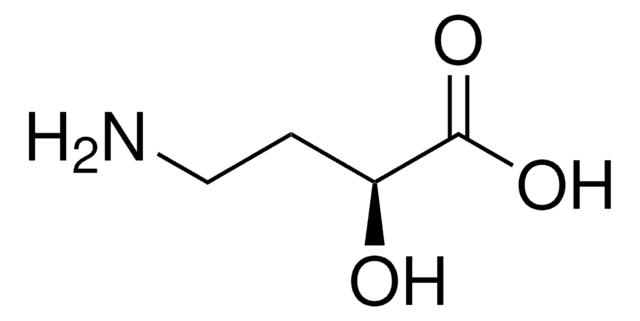
4-Amino-3-hydroxybutyric acid
98%
Synonym(s):
DL-γ-Amino-β-hydroxybutyric acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
H2NCH2CH(OH)CH2CO2H
CAS Number:
Molecular Weight:
119.12
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder or crystals
reaction suitability
reaction type: solution phase peptide synthesis
color
white to yellow
mp
223 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
NCC(O)CC(O)=O
InChI
1S/C4H9NO3/c5-2-3(6)1-4(7)8/h3,6H,1-2,5H2,(H,7,8)
InChI key
YQGDEPYYFWUPGO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D Jordan et al.
Brain research, 268(1), 105-110 (1983-05-23)
Various doses of GABA from 0.25 to 5 mumol injected into the third ventricle decrease serum TSH rapidly. The same effect was observed with GABOB (10 mumol), the hydroxylated form of GABA. The inhibitory effect of both of these drugs
[Neurochemical aspects of the pharmacology of GABAergic substances].
K S Raevskiĭ
Farmakologiia i toksikologiia, 44(5), 517-529 (1981-09-01)
T W Stone
European journal of pharmacology, 128(1-2), 81-83 (1986-08-22)
Kynurenine and kynurenic acid are known to produce convulsions in rats and mice and it has been reported that kynurenine can displace GABA from its neuronal binding sites. The present study shows that neither kynurenine nor kynurenic acid are antagonist
M Candela et al.
Journal of chromatography. A, 890(2), 273-280 (2000-09-29)
A rapid and simple reversed-phase liquid chromatographic method that did not require the derivatization of 4-amino-3-hydroxybutyric acid (GABOB) was developed and validated. The method proved to be suitable for the determination of GABOB concentrations in finished pharmaceutical product (tablets). The
T Noto et al.
Journal of neurochemistry, 51(2), 548-551 (1988-08-01)
gamma-Amino-beta-[3H]hydroxybutyric acid ([3H]-GABOB) was formed in rat brain from 2-[3H]-hydroxyputrescine that had been chemically synthesized from 2-oxoputrescine and [3H]sodium borohydride. After the injection of 2-[3H]hydroxyputrescine into the lateral ventricle of a rat brain, the rat was killed and then the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service