All Photos(2)
About This Item
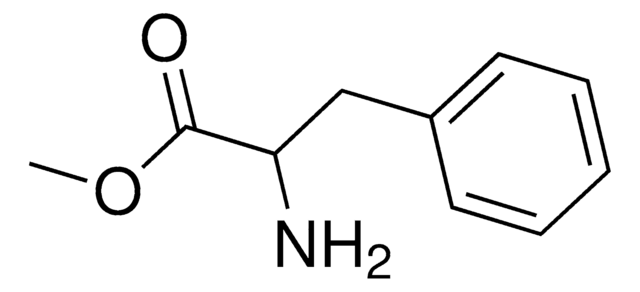
Linear Formula:
C6H5CH2CH(NH2)CO2CH3 · HCL
CAS Number:
Molecular Weight:
215.68
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
reaction suitability
reaction type: solution phase peptide synthesis
mp
159-163 °C (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
Cl[H].COC(=O)[C@H](N)Cc1ccccc1
InChI
1S/C10H13NO2.ClH/c1-13-10(12)9(11)7-8-5-3-2-4-6-8;/h2-6,9H,7,11H2,1H3;1H/t9-;/m1./s1
InChI key
SWVMLNPDTIFDDY-SBSPUUFOSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
C S Rosenfeld
Blood, 80(9), 2401-2405 (1992-11-01)
Phenylalanine methylester (PME), a lysosomotropic compound can be used to deplete monocytes and myeloid cells from peripheral blood and bone marrow (BM). The potential of PME for purging leukemic cells from BM was investigated using U937 and HL-60 cell lines
P L Triozzi et al.
Immunopharmacology, 28(1), 39-45 (1994-07-01)
Monocytes macrophages have negative regulatory effects on many immunologic responses. Depletion of monocytes from peripheral blood using the lysosomotropic agent, L-phenylalanine methyl ester (PME), has been shown to improve lymphokine activated killer (LAK) cell expansion in vitro. A pilot study
Hua Fu et al.
Chemical communications (Cambridge, England), (1)(1), 134-135 (2003-03-04)
Reaction of ADP with amino acid methyl esters mediated by trimethylsilyl chloride in pyridine produced adenosine 5'-phosphoramidates in good yields under mild conditions, it is interesting that nucleophilic attack of amino acid methyl esters only occurred on alpha-phosphorus of ADP.
R D Skwierczynski et al.
Pharmaceutical research, 10(8), 1174-1180 (1993-08-01)
The kinetics of demethylation of aspartame and L-phenylalanine methyl ester were studied in aqueous solution at 25 degrees C over the pH range 0.27-11.5. The pseudo-first-order rate constant for aspartame was resolved into individual contributions from methyl ester hydrolysis and
S Reissmann et al.
Journal of medicinal chemistry, 39(4), 929-936 (1996-02-16)
For further studies on the structural and conformational requirements of positions 2,3, and 7 in the bradykinin sequence, we replaced the proline residues by the more hydrophobic and conformationally restricted N-methyl-L- and D-phenylalanine (NMF). The biological activities of the new
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service