488216
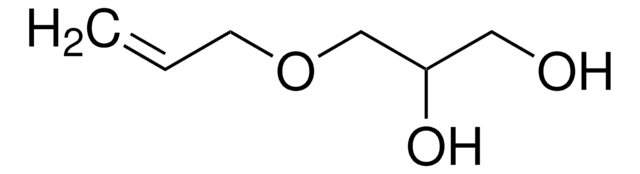
3,4-Dihydroxy-1-butene
≥99%
Synonym(s):
3-Butene-1,2-diol
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH2=CHCH(OH)CH2OH
CAS Number:
Molecular Weight:
88.11
Beilstein:
1633578
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥99%
bp
195 °C/733 mmHg (lit.)
density
1.047 g/mL at 25 °C (lit.)
functional group
hydroxyl
SMILES string
OCC(O)C=C
InChI
1S/C4H8O2/c1-2-4(6)3-5/h2,4-6H,1,3H2
InChI key
ITMIAZBRRZANGB-UHFFFAOYSA-N
Related Categories
General description
3,4-Dihydroxy-1-butene, also known as 3-butene-1,2-diol (BDdiol), is a metabolite of 1,3-butadiene. It forms the precursor for synthesizing different chiral building blocks. BDdiol can undergo oxidation to form hydroxymethylvinyl ketone (HMVK). 1,2-epoxy-3-butene (EB) on hydrolysis in the presence of epoxide hydrolases (EH) forms BDdiol.
Application
3,4-Dihydroxy-1-butene can be used:
- As a reactant to synthesize cyclic organic carbonates by continuous flow procedure.
- To prepare substituted oxazolidinone ligands used to target medicinally relevant RNAs.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Versatile and scalable synthesis of cyclic organic carbonates under organocatalytic continuous flow conditions
Gerardy R, et al.
Catalysis Science & Technology, 9(24), 6841-6851 (2019)
Gregory M Sandala et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 15(19), 4865-4873 (2009-03-28)
High-level conventional ab initio and density functional theory (DFT) calculations have been performed to examine the fate of the native substrate glycerol (1) and its analogue but-3-ene-1,2-diol (7) in the coenzyme B(12)-dependent enzyme glycerol dehydratase (GDH). Experimental studies find that
X Cheng et al.
Drug metabolism and disposition: the biological fate of chemicals, 21(1), 121-124 (1993-01-01)
A rapid, simple extraction and GC assay procedure is described that allows quantitation of micromolar concentrations of butadiene bisoxide and 3-butene-1,2-diol in microsomal suspensions exposed to butadiene. Butane-1,4-diol is used as the internal standard. The recovery of these compounds from
R J Krause et al.
Chemical research in toxicology, 14(12), 1590-1595 (2001-12-18)
The metabolic fate of 3-butene-1,2-diol (BDD), a secondary metabolite of the industrial carcinogen, 1,3-butadiene, is unclear. The current study characterizes BDD oxidation to hydroxymethylvinyl ketone (HMVK), a reactive Michael acceptor. Because of its instability in aqueous medium, HMVK was trapped
E Malvoisin et al.
Xenobiotica; the fate of foreign compounds in biological systems, 12(2), 137-144 (1982-02-01)
1. In rat liver microsomes, 1,3-butadiene was metabolized to butadiene monoxide, which was subsequently transformed into 3-butene-1,2-diol by microsomal epoxide hydrolase. 2. In the metabolism of butadiene oxide in microsomes, four metabolites were detected, namely two stereoisomers of DL-diepoxybutane, and
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service