All Photos(2)
About This Item
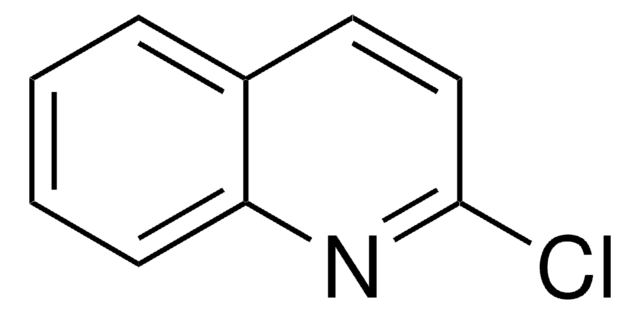
Empirical Formula (Hill Notation):
C8H6N2O
CAS Number:
Molecular Weight:
146.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99% (HPLC)
mp
271-272 °C (lit.)
SMILES string
Oc1cnc2ccccc2n1
InChI
1S/C8H6N2O/c11-8-5-9-6-3-1-2-4-7(6)10-8/h1-5H,(H,10,11)
InChI key
FFRYUAVNPBUEIC-UHFFFAOYSA-N
General description
Epitaxial crystallization of syndiotactic polypropylene on 2-quinoxalinol yields isochiral form II of syndiotactic polypropylene. 2-Quinoxalinol participates in direct dehydrative cross-coupling of 2-quinoxalinone with p-tolylacetylene via Pd/Cu-catalyzed phosphonium coupling.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fu-An Kang et al.
Chemical communications (Cambridge, England), 46(8), 1347-1349 (2010-05-08)
The first chemoselective direct dehydrative cross-coupling of tautomerizable heterocycles with alkynes has been achieved via C-H/C-OH bond activations with direct C(sp(2))-C(sp) bond formation, which is in line with ideal synthesis using readily available materials.
Isochiral form II of syndiotactic polypropylene produced by epitaxial crystallization.
Zhang J, et al.
Macromolecules, 34(18), 6261-6267 (2001)
Pramila Menon et al.
Chemosphere, 53(8), 1023-1031 (2003-09-25)
The dissipation of 14C carbaryl in undisturbed soil cores, and of quinalphos (25EC and 20AF) after seed and soil treatments, was investigated under field use conditions, in a semi-arid groundnut field. Residues were analyzed by TLC and HPLC and additionally
Andreas Behrends et al.
Redox report : communications in free radical research, 9(5), 279-288 (2004-12-21)
Toxicity of the pesticide quinalphos may comprise secondary, delayed effects by its main metabolite 2-hydroxyquinoxaline (HQO). We demonstrate that HQO can destroy photocatalytically vitamins C and E, catecholamines, serotonin, melatonin, the melatonin metabolite AMK (N(1)-acetyl-5-methoxykynuramine), and unsubstituted and substituted anthranilic
R Q He et al.
Biochemistry and molecular biology international, 37(3), 447-457 (1995-10-01)
A procedure for transaminating proteins and removing the transaminated N-terminal residue has been used for studying structure-function relationship of protein (Dixon and Fields 1972, Meth. Enzymol. 25, 409-419). We show that it is convenient for measuring the relative molecular masses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service