P38706
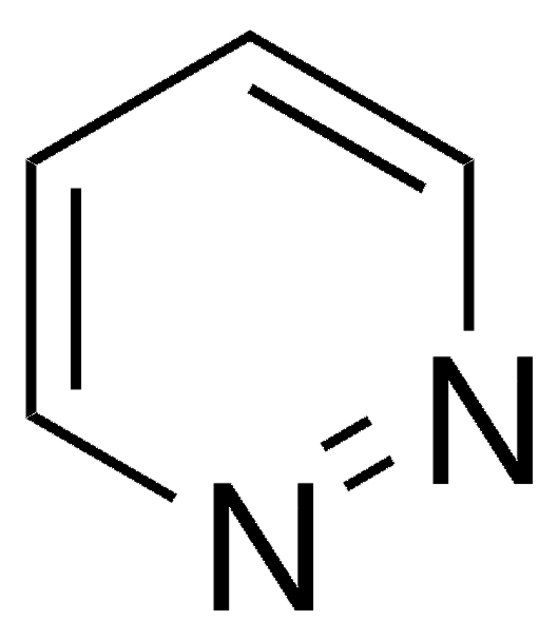
Phthalazine
98%
Synonym(s):
β-Phenodiazine, 2,3-Benzodiazine, 2,3-Diazanaphthalene, Benzo[d]pyridazine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C8H6N2
CAS Number:
Molecular Weight:
130.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
189 °C/29 mmHg (lit.)
mp
89-92 °C (lit.)
storage temp.
2-8°C
SMILES string
c1ccc2cnncc2c1
InChI
1S/C8H6N2/c1-2-4-8-6-10-9-5-7(8)3-1/h1-6H
InChI key
LFSXCDWNBUNEEM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Muta. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hyungchul Kim et al.
International journal of pharmaceutics, 377(1-2), 105-111 (2009-05-26)
Investigation of the use of solution NMR spectroscopy to determine the effect of organic solvents on chemical shift changes in bases on addition of acids is reported. This information can be useful in the evaluation of solvents and counterion selection
Jin Sung Kim et al.
Bioorganic & medicinal chemistry, 16(8), 4545-4550 (2008-03-07)
Studies on antitumor heterocyclic quinones containing nitrogens revealed that the number and position of nitrogens on the heterocyclic ring have significance on cytotoxicity of quinones. In our continuous effort to find more cytotoxic quinone compounds, we designed triazolophthalazine analogues in
Kebin Li et al.
Analytical chemistry, 80(13), 4945-4950 (2008-05-30)
Arrays of Au nanowells (NWs) were fabricated by electron-beam lithography (EBL) and characterized by surface plasmon resonance (SPR) and surface-enhanced Raman scattering (SERS). It is revealed that these Au NW arrays exhibit multiple SP resonances that can be tuned by
Brad Herberich et al.
Journal of medicinal chemistry, 51(20), 6271-6279 (2008-09-27)
Investigations into the structure-activity relationships (SAR) of a series of phthalazine-based inhibitors of p38 are described. These efforts originated from quinazoline 1 and through rational design led to the development of a series of orally bioavailable, potent, and selective inhibitors.
R S K Vijayan et al.
Journal of molecular graphics & modelling, 27(3), 286-298 (2008-06-21)
Given the heterogeneity of GABA(A) receptor, the pharmacological significance of identifying subtype selective modulators is increasingly being recognized. Thus, drugs selective for GABA(A) alpha(3) receptors are expected to display fewer side effects than the drugs presently in clinical use. Hence
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service