All Photos(1)
About This Item
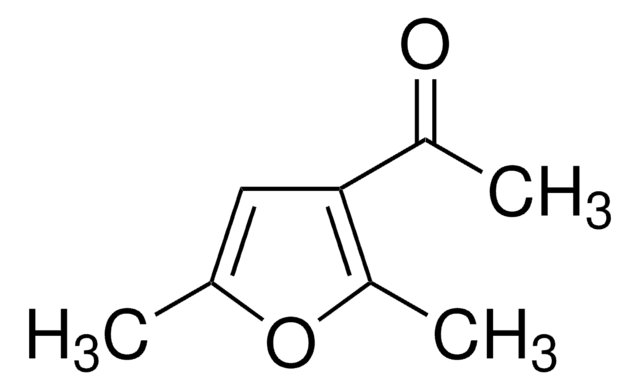
Empirical Formula (Hill Notation):
C8H10OS
CAS Number:
Molecular Weight:
154.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.544 (lit.)
bp
105-108 °C/15 mmHg (lit.)
density
1.086 g/mL at 25 °C (lit.)
functional group
ketone
SMILES string
CC(=O)c1cc(C)sc1C
InChI
1S/C8H10OS/c1-5-4-8(6(2)9)7(3)10-5/h4H,1-3H3
InChI key
PUSJAEJRDNPYKM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
3-Acetyl-2,5-dimethylthiophene was used in the synthesis of heterocyclic ketimines, 3-acetyl-2,5-dimethylthiophene thiosemicarbazone and 3-acetyl-2,5- dimethylthiophene semicarbazone.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Krishna Sharma et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 75(1), 422-427 (2009-12-08)
Reactions of 3-acetyl-2,5-dimethylthiophene with thiosemicarbazide and semicarbazide hydrochloride resulted in the formation of new heterocyclic ketimines, 3-acetyl-2,5-dimethylthiophene thiosemicarbazone (C(9)H(13)N(3)OS(2) or L(1)H) and 3-acetyl-2,5- dimethylthiophene semicarbazone (C(9)H(13)N(3)OS or L(2)H), respectively. The Pd(II) and Pt(II) complexes have been synthesized by mixing metal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service