P73404
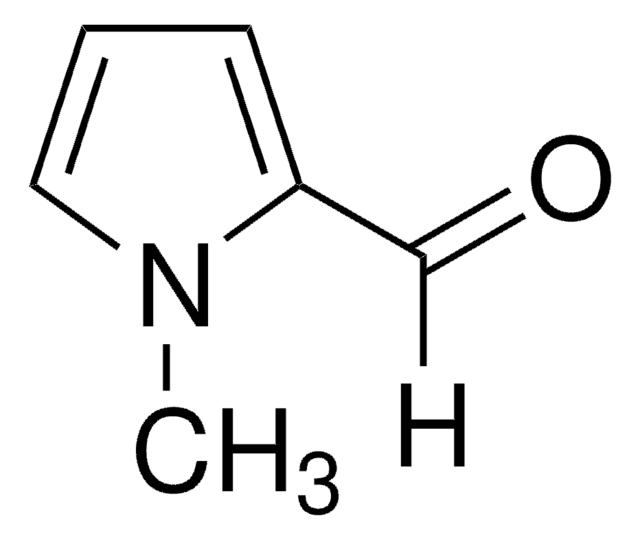
Pyrrole-2-carboxaldehyde
98%
Synonym(s):
2-Formylpyrrole
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C5H5NO
CAS Number:
Molecular Weight:
95.10
Beilstein:
105745
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
bp
217-219 °C (lit.)
mp
43-46 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]C(=O)c1ccc[nH]1
InChI
1S/C5H5NO/c7-4-5-2-1-3-6-5/h1-4,6H
InChI key
ZSKGQVFRTSEPJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Pyrrole-2-carboxaldehydesis a heterocyclic building blocks characterized by a pyrrole ring with a formylgroup attached at the 2-position used in the production of various biologicallyactive compounds. Highly functionalized pyrrole-2-carboxaldehydes have beenutilized as an intermediate in the creation of oligopyrrole macrocycles.
Application
- Pyrimidine-based functional fluorescent organic nanoparticle probe for detection of Pseudomonas aeruginosa.: This study used pyrrole-2-carboxaldehyde to develop a fluorescent nanoparticle probe based on pyrimidine for detecting Pseudomonas aeruginosa, enhancing diagnostic capabilities in microbiology (Kaur G et al., 2015).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
224.6 °F - closed cup
Flash Point(C)
107 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Z H Chohan et al.
Chemical & pharmaceutical bulletin, 40(9), 2555-2556 (1992-09-01)
A new Schiff-base ligand N-(2'-pyrrylmethylidene)2-aminopyrimidine derived from the reaction of 2-amino pyrimidine and pyrrol-2-carboxaldehyde and its nickel(II), copper(II) and zinc(II) complexes have been synthesised and characterised on the basis of elemental analysis, molar conductance, infrared, electronic and proton nuclear magnetic
Investigation of optical and electrical properties of Pyrrole-2- carboxyldehyde (PCL) in PVA polymer matrix
Rana, Meenakshi and Devlal, et al.
Materials Today: Proceedings, 49, 3279-3282 (2022)
Huajian Zhu et al.
Organic letters, 13(10), 2792-2794 (2011-04-23)
A new synthetic protocol for efficient and regiospecifc assembly of indolizines and pyrido[1,2-a]indoles by coupling of substituted methyl bromides and alkynes with corresponding pyrrole-2-carboxaldehyde and 1H-indole-2-carboxaldehyde has been developed. Additionally, a possible mechanism for the reaction is proposed.
Aaron R Coffin et al.
The Journal of organic chemistry, 71(17), 6678-6681 (2006-08-12)
A regiocontrolled synthesis of 3,4-disubstituted pyrrole-2-carboxaldehydes was completed in two steps from acyclic starting materials. A Barton-Zard pyrrole synthesis between N-methoxy-N-methyl-2-isocyanoacetamide and alpha-nitroalkenes or beta-nitroacetates provided N-methoxy-N-methyl pyrrole-2-carboxamides (pyrrole Weinreb amides), which were converted into the corresponding pyrrole-2-carboxaldehydes by treatment
Barbara Michela Giuliano et al.
The journal of physical chemistry. A, 114(7), 2506-2517 (2010-01-30)
Monomeric pyrrole-2-carbaldehyde (P2C) was isolated in low-temperature argon and xenon matrices, and its UV-induced photochemistry was studied. The structures of the reagent as well as the reaction photoproducts were characterized by FTIR spectroscopy. Interpretation of the experimental results was assisted
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service