All Photos(1)
About This Item
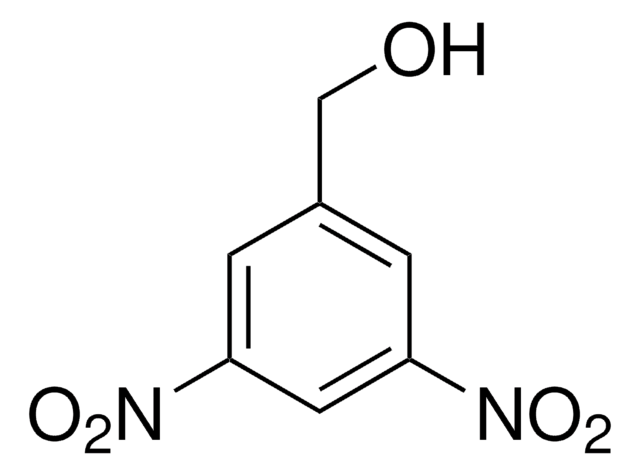
Linear Formula:
O2NC6H4CH2OH
CAS Number:
Molecular Weight:
153.14
Beilstein:
2044769
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
bp
175-180 °C/3 mmHg (lit.)
mp
26-32 °C (lit.)
density
1.29 g/mL at 20 °C (lit.)
functional group
hydroxyl
nitro
SMILES string
OCc1cccc(c1)[N+]([O-])=O
InChI
1S/C7H7NO3/c9-5-6-2-1-3-7(4-6)8(10)11/h1-4,9H,5H2
InChI key
CWNPOQFCIIFQDM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
3-Nitrobenzyl alcohol is the best substrate for cytosolic alcohol dehydrogenase.
Application
3-Nitrobenzyl alcohol was employed as matrix during fast-atom bombardment mass spectrometry. It was used in isolation of palmitylated peptide fragment from bovine rhodopsin and its characterization by mass spectrometry.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Charlotte Mallet et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 21(21), 7944-7953 (2015-04-14)
A series of 2,5-distyrylfuran derivatives bearing pentafluorophenyl- and cyanovinyl units have been synthesized for aggregation-induced emission (AIE). The effect of the type and extent of the supramolecular connections on the AIE of the furan derivatives were examined and correlated with
D I Papac et al.
The Journal of biological chemistry, 267(24), 16889-16894 (1992-08-25)
Bovine rhodopsin has been reported to be S-palmitylated at cysteines 322 and 323 (Ovchinnikov, Y. A., Abdulaev, N. G., and Bogachuk, A.S. (1988) FEBS Lett. 230, 1-5). Using a combination of enzymatic and chemical cleavage techniques in conjunction with tandem
Harry J Sterling et al.
Journal of the American Society for Mass Spectrometry, 20(10), 1933-1943 (2009-08-18)
The use of m-nitrobenzyl alcohol (m-NBA) to enhance charging of noncovalent complexes formed by electrospray ionization from aqueous solutions was investigated. Addition of up to 1% m-NBA can result in a significant increase in the average charging of complexes, ranging
R S Pappas et al.
Carbohydrate research, 197, 1-14 (1990-03-25)
A derivatization method, adapted from that of Angel et al. (ref. 10), for sequencing sugar residues in partially degraded poly- and oligo-saccharides using positive-ion f.a.b.-m.s. is described. Derivative selection provides sequence information by directing fragmentation exclusively to both sides of
Yuya Domoto et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 20(48), 15998-16005 (2014-10-07)
Efficient end-capping synthesis of neutral donor-acceptor (D-A) [2]rotaxanes without loading any catalysts or activating agents was achieved by utilizing high reactivity of a pentacoordinated hydrosilane toward salicylic acid derivatives. As components of [2]rotaxanes, an electron-deficient naphthalenediimide-containing axle with a salicylic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service