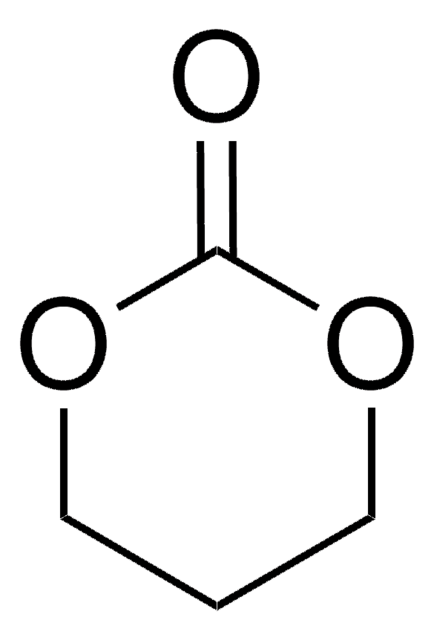
455067
4-(Hydroxymethyl)-1,3-dioxolan-2-one
Synonym(s):
(2-Oxo-1,3-dioxolan-4-yl)methanol, 3-Hydroxypropene carbonate, 3-Hydroxypropylene carbonate, 4-Methylolethylene carbonate, Glycerin carbonate, Glycerol 1,2-carbonate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O4
CAS Number:
Molecular Weight:
118.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.469 (lit.)
Quality Level
bp
137-140 °C/0.5 mmHg (lit.)
density
1.4 g/mL at 25 °C (lit.)
SMILES string
OCC1COC(=O)O1
InChI
1S/C4H6O4/c5-1-3-2-7-4(6)8-3/h3,5H,1-2H2
InChI key
JFMGYULNQJPJCY-UHFFFAOYSA-N
Related Categories
Application
4-(Hydroxymethyl)-1,3-dioxolan-2-one can be used as a building block to synthesize:
- 4-[(prop-2-en-1-yloxy)methyl]-1,3-dioxolan-2-one (AGC) via Williamson ether synthesis with 3-bromoprop-1-ene.
- Hyperbranched polyethers via copolymerization with cyclic carbonate containing phthalimide moieties.
- (2-Oxo-1,3-dioxolan-4-yl)methyl benzeneacetate by reacting with phenylacetyl chloride.
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Polyurethanes with pendant hydroxyl groups: synthesis and characterization.
Ubaghs L, et al.
Macromolecular Rapid Communications, 25(3), 517-521 (2004)
Converting wastes into added value products: from glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes.
Dibenedetto A, et al.
Tetrahedron, 67(6), 1308-1313 (2011)
Synthesis of glycerin carbonate-based intermediates using thiol-ene chemistry and isocyanate free polyhydroxyurethanes therefrom
Benyahya S, et al.
Polym. Chem., 2(11), 2661-2667 (2011)
Amine functionalized polyglycerols obtained by copolymerization of cyclic carbonate monomers
Parzuchowski PG, et al.
Polymer, 151, 250-260 (2018)
Cyclic carbonates obtained by reactions of alkali metal carbonates with epihalohydrins.
Rokicki G and Kuran W.
Bulletin of the Chemical Society of Japan, 57(6), 1662-1666 (1984)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service