137952
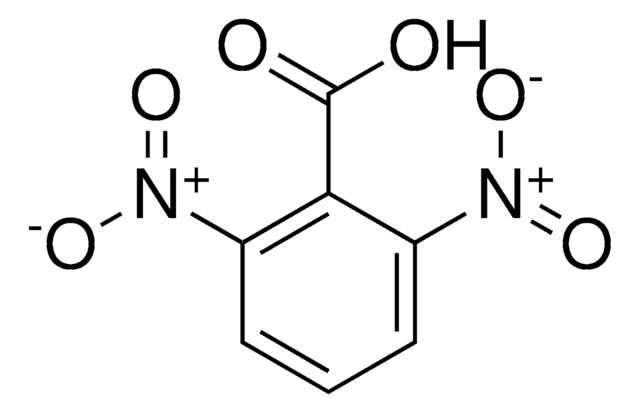
2,4-Dinitrobenzoic acid
96%
Synonym(s):
1-Carboxy-2,4-dinitrobenzene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(O2N)2C6H3CO2H
CAS Number:
Molecular Weight:
212.12
Beilstein:
658650
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
mp
176-180 °C (lit.)
functional group
carboxylic acid
nitro
SMILES string
OC(=O)c1ccc(cc1[N+]([O-])=O)[N+]([O-])=O
InChI
1S/C7H4N2O6/c10-7(11)5-2-1-4(8(12)13)3-6(5)9(14)15/h1-3H,(H,10,11)
InChI key
ZIIGSRYPZWDGBT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2,4-Dinitrobenzoic acid can be used as a reactant:
It can also be used in the spectrophotometric determination of diazepam in pure samples and in its pharmaceutical preparations. In capillary zone electrophoresis, it can be employed as chromophore probe for the analysis of perfluorinated carboxylic acids in water.
- In decarboxylative C-N cross-coupling reactions.
- To synthesize Zwitterionic azaspirocyclic hydantoins by reacting with various carbodiimides via in situ intramolecular dearomatization reaction.
- To prepare 1-(2,4-dinitrophenyl)ethanone by condensation with dimethyl malonate and subsequent decarboxylation reaction.
It can also be used in the spectrophotometric determination of diazepam in pure samples and in its pharmaceutical preparations. In capillary zone electrophoresis, it can be employed as chromophore probe for the analysis of perfluorinated carboxylic acids in water.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
CuSO4-mediated decarboxylative C-N cross-coupling of aromatic carboxylic acids with amides and anilines
Sheng W-J, et al.
Tetrahedron Letters, 56(4), 599-601 (2015)
Spectrophotometric determination of diazepam in pure form, tablets and ampoules
El-Hawary WF, et al.
International Journal of Biomedical Science : IJBS, 3(1), 50-50 (2007)
Photolysis of dinitrobenzyl alcohols, dinitrobenzaldehydes, and nitrobenzoic acids in seawater, estuary water, and pure water.
Prak DJL, et al.
Marine Chemistry, 145-147, 29-36 (2012)
Bicyclic heterocyclic anthranilic diamides as ryanodine receptor modulators with insecticidal activity
Jeanguenat A, et al.
Bioorganic & Medicinal Chemistry, 24(3), 403-427 (2016)
W F El-Hawary et al.
International journal of biomedical science : IJBS, 3(1), 50-55 (2007-03-01)
The interaction of diazepam with picric acid (I), 3,5-dinitrobenzoic acid (II) and 2,4-dinitrobenzoic acid (III) was found to be useful for its spectrophotometric determination. The quantitation was carried out at 475, 500, and 500 nm for the reaction with (I)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service