180246
2-Naphthoic acid
98%
Synonym(s):
2-Naphthalenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C10H7CO2H
CAS Number:
Molecular Weight:
172.18
Beilstein:
972039
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
mp
185-187 °C (lit.)
solubility
alcohol: soluble
diethyl ether: soluble
hot water: slightly soluble
functional group
carboxylic acid
SMILES string
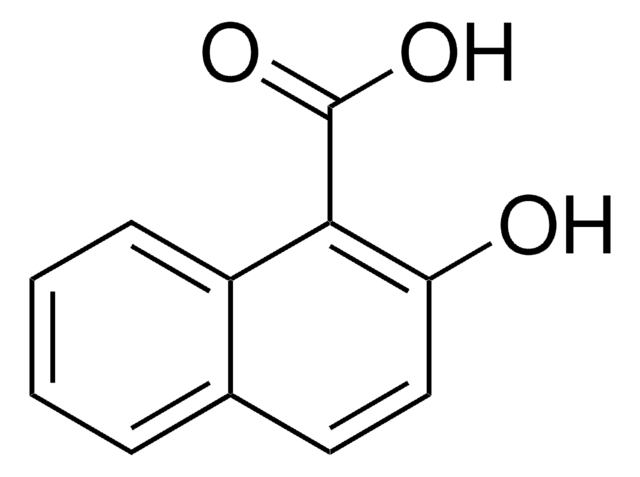
OC(=O)c1ccc2ccccc2c1
InChI
1S/C11H8O2/c12-11(13)10-6-5-8-3-1-2-4-9(8)7-10/h1-7H,(H,12,13)
InChI key
UOBYKYZJUGYBDK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Naphthoic acid (NPA) is a noncompetitive N-methyl-D-aspartate (NMDA) receptor inhibitor. The fluorescence spectra and electronic absorption of 2-naphthoic acid was studied.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Han Yu et al.
Molecular pharmacology, 84(4), 541-550 (2013-07-23)
N-Methyl-D-aspartate (NMDA) receptors mediate excitatory synaptic transmission in the central nervous system and play important roles in synaptic development and plasticity, but also mediate glutamate neurotoxicity. Recently, 2-naphthoic acid (NPA) and its derivatives have been identified as allosteric, noncompetitive NMDA
An electronic spectral study of the influence of thermal and electronic processes on the determination of the excited singlet-state dissociation constants of 1-and 2-naphthoic acid.
Kovi PJ and Schulman SG.
Analytica Chimica Acta, 63(1), 39-52 (1973)
K L Yu et al.
Journal of medicinal chemistry, 39(12), 2411-2421 (1996-06-07)
In search for retinoic acid receptor (RAR) selective ligands, a series of 6-substituted 2-naphthoic acid retinoids were synthesized and evaluated in vitro in a transactivation assay and a competition binding assay for all RARs. These derivatives, in general, showed RAR
D P McNamara et al.
Journal of pharmaceutical sciences, 75(9), 858-868 (1986-09-01)
A mass transfer model was developed to describe the dissolution and reaction of acidic and basic compounds from a rotating disk in unbuffered water. Dissolution of two carboxylic acids, 2-naphthoic acid (1) and naproxen [(+)-6-methoxy-alpha-methyl-2-naphthaleneacetic acid, 2], and the free
Toshiki Furuya et al.
ChemSusChem, 2(7), 645-649 (2009-06-30)
The large pool of cytochrome P450 (P450) open-reading frames identified in genome sequences has attracted much attention as a resource for new oxidation biocatalysts. P450 genes were cloned from genome-sequenced bacteria and coexpressed with putidaredoxin and its reductase genes to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service