112968
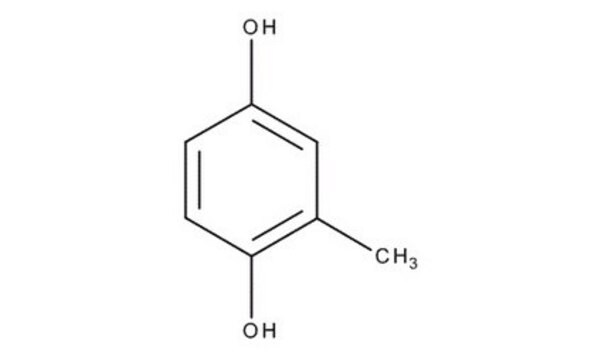
Methylhydroquinone
99%
Synonym(s):
Toluhydroquinone
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
CH3C6H3-1,4-(OH)2
CAS Number:
Molecular Weight:
124.14
Beilstein:
2041489
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
solid
autoignition temp.
851 °F
mp
128-130 °C (lit.)
SMILES string
Cc1cc(O)ccc1O
InChI
1S/C7H8O2/c1-5-4-6(8)2-3-7(5)9/h2-4,8-9H,1H3
InChI key
CNHDIAIOKMXOLK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Methylhydroquinone is produced by the oxidation of o-cresol by the mutants G103S, G103S/A107G, and G103S/A107T.
Application
Methylhydroquinone can be used as a reactant to prepare:
- A semiflexible thermotropic polyester via polycondensation reaction with 4,4′-sebacoyldioxydibenzoyl chloride.
- A sesquiterpene (±)-helibisabonol A.
- poly{hexakis[(methyl)(4-hydroxyphenoxy)]cyclotriphosphazene} by reacting with hexachlorocyclotriphosphazene.
- 6-Hydroxy-4,7-dimethyl-2H-1-benzopyran-2-one by treating with ethyl acetoacetate in the presence of H2SO4 as a catalyst.
Biochem/physiol Actions
Methylhydroquinone (Toluquinol) inhibits the growth of endothelial and tumor cells in culture in the micromolar range and is a promising drug candidate in the treatment of cancer and other angiogenesis-related pathologies.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Corr. 1A - Skin Sens. 1 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
Flash Point(F)
341.6 °F - closed cup
Flash Point(C)
172 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and mesomorphic properties of a semiflexible polyester based on 4, 4?-sebacoyldioxydibenzoyl chloride and methylhydroquinone
Costa Giovanna, et al.
Macromolecular Chemistry and Physics, 191(4), 791-800 (1990)
Ying Tao et al.
Journal of bacteriology, 186(14), 4705-4713 (2004-07-03)
Wild-type toluene 4-monooxygenase (T4MO) of Pseudomonas mendocina KR1 oxidizes toluene to p-cresol (96%) and oxidizes benzene sequentially to phenol, to catechol, and to 1,2,3-trihydroxybenzene. In this study T4MO was found to oxidize o-cresol to 3-methylcatechol (91%) and methylhydroquinone (9%), to
Synthesis and characterization of poly {hexakis [(methyl)(4-hydroxyphenoxy)] cyclotriphosphazene}
Luther T A, et al.
Journal of Applied Polymer Science, 82(14), 3439-3446 (2001)
First total synthesis of ?-helibisabonol A
Macias F A, et al.
Tetrahedron Letters, 43(36), 6417-6420 (2002)
Solvent-Free Synthesis and Insecticidal Activities of C6-Esterified Coumarins
Xie Ying, et al.
Letters in Drug Design & Discovery, 11(9), 1124-1132 (2014)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service