C2932
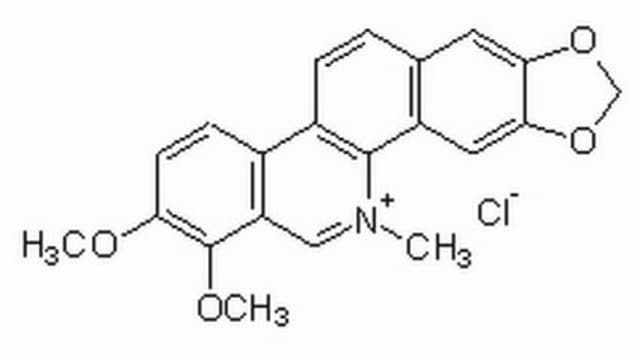
Chelerythrine chloride
≥95% (TLC), powder
Sinónimos:
1,2-Dimethoxy-N-methyl(1,3)benzodioxolo(5,6-c)phenanthridinium chloride, Toddaline chloride
About This Item
Productos recomendados
origen biológico
plant
Nivel de calidad
Ensayo
≥95% (TLC)
Formulario
powder
color
yellow to orange
mp
213.0-214.0 °C
solubilidad
DMSO: 2 mg/mL
temp. de almacenamiento
−20°C
cadena SMILES
Cl.COc1ccc2-c3ccc4cc5OCOc5cc4c3[N](C)=Cc2c1OC
InChI
1S/C21H18NO4.ClH/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22;/h4-10H,11H2,1-3H3;1H
Clave InChI
SUPBMPBJXZDANZ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- as a supplement in heat-inactivated late-embryo extract or late-embryo extract to inhibit protein kinase C (PKC) activity
- for in vitro Xenopus experiments
- as a PKC inhibitor in HL-1 cells, to block the PKC pathway to study its effects on doxazosin-induced galectin-3 and collagen expression
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico