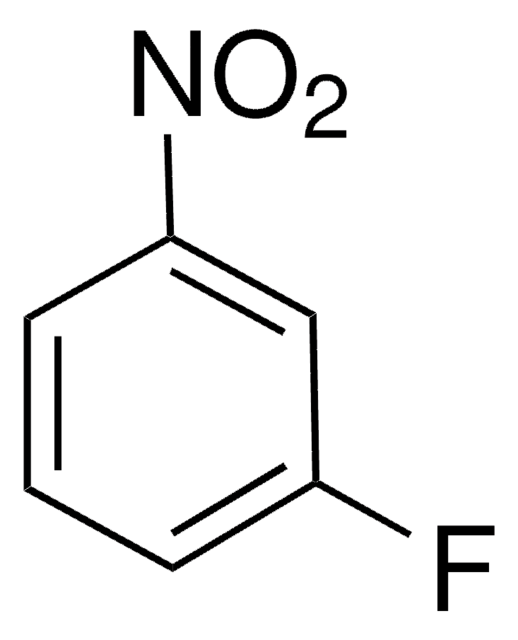
71210
Sodium ethoxide
technical, ≥95% (T)
Sinónimos:
Sodium ethylate
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3CH2ONa
Número de CAS:
Peso molecular:
68.05
Beilstein/REAXYS Number:
3593646
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.21
Productos recomendados
vapor density
1.6 (vs air)
Quality Level
vapor pressure
<0.1 mmHg ( 20 °C)
grade
technical
assay
≥95% (T)
form
powder
impurities
~2% Na2CO3 and NaOH
SMILES string
[Na+].CC[O-]
InChI
1S/C2H5O.Na/c1-2-3;/h2H2,1H3;/q-1;+1
InChI key
QDRKDTQENPPHOJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Sodium ethoxide (Sodium ethylate) is a sodium alkoxide. It has been synthesized by reacting sodium with ethanol. It undergoes decomposition in the presence of water to afford ethanol and sodium hydroxide. It is widely employed as a strong base in organic synthesis studies.
Sodium ethoxide is an alkoxide salt mainly used as a strong base in organic reactions such as deprotonation, dehydration and dehalogenation.
Application
Sodium ethoxide may be used as a base for the palladium catalyzed cross-coupling of aryl halides and alkenylboranes to synthesize arylated (E)-alkenes.
Sodium ethoxide may be used for the preparation of tricarbonylchloro(glycinato)ruthenium(II) (CORM-3).
signalword
Danger
hcodes
Hazard Classifications
Flam. Sol. 1 - Self-heat. 1 - Skin Corr. 1A
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 1
flash_point_f
86.0 °F - closed cup
flash_point_c
30 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Whitaker KS and Whitaker DT
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Stereoselective synthesis of arylated (E)-alkenes by the reaction of alk-1-enylboranes with aryl halides in the presence of palladium catalyst.
Miyaura N & Suzuki, A.
Journal of the Chemical Society. Chemical Communications, 19, 866-867 (1979)
James E Clark et al.
Circulation research, 93(2), e2-e8 (2003-07-05)
Carbon monoxide, which is generated in mammals during the degradation of heme by the enzyme heme oxygenase, is an important signaling mediator. Transition metal carbonyls have been recently shown to function as carbon monoxide-releasing molecules (CO-RMs) and to elicit distinct
Eagleson M.
Concise Encyclopedia Chemistry, 997-997 (1994)
On the preparation of optically active phenyl-p-tolyl-methanesulphonic acid and its racemisation by sodium ethoxide.
E E PEDERSEN et al.
Acta chemica Scandinavica, 2(8), 651-656 (1948-01-01)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico