32824
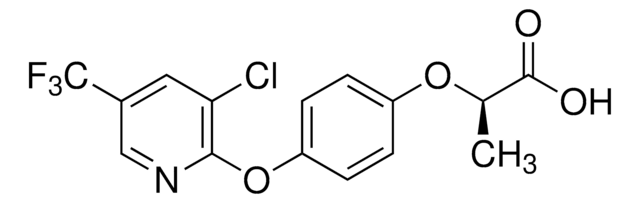
Fluazifop
PESTANAL®, analytical standard
Sinónimos:
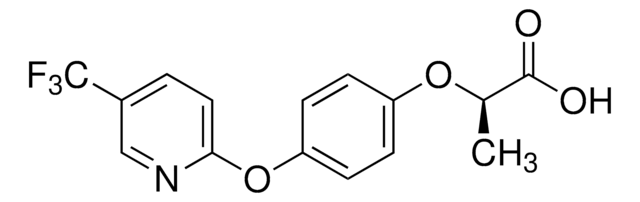
2-[4-(5-Trifluoromethyl-2-pyridyloxy)phenoxy]propionic acid
About This Item
Productos recomendados
grade
analytical standard
Quality Level
product line
PESTANAL®
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
application(s)
agriculture
environmental
format
neat
SMILES string
CC(Oc1ccc(Oc2ccc(cn2)C(F)(F)F)cc1)C(O)=O
InChI
1S/C15H12F3NO4/c1-9(14(20)21)22-11-3-5-12(6-4-11)23-13-7-2-10(8-19-13)15(16,17)18/h2-9H,1H3,(H,20,21)
InChI key
YUVKUEAFAVKILW-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Rainwater samples by solid-phase extraction (SPE) and liquid chromatography-tandem mass spectrometry (LC-MS/MS).
- Vegetables by acetonitrile-based QuEChERS (quick, easy, cheap, effective, rugged and safe) extraction followed by LC-MS/MS.
Recommended products
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Aquatic Acute 1 - Repr. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico