901251
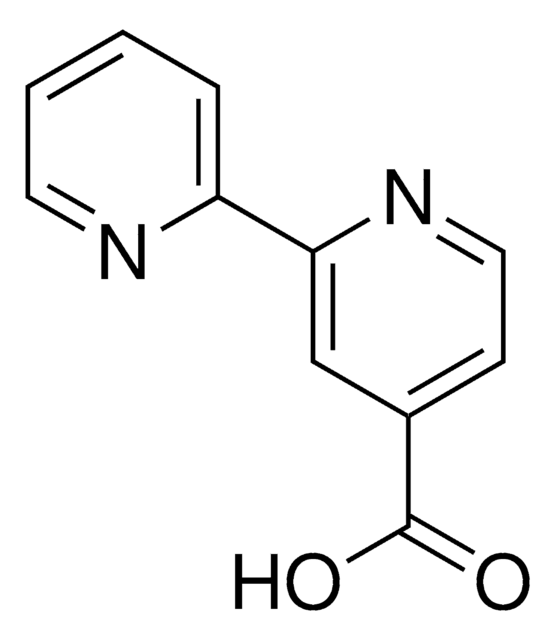
[2,2′-Bipyridine]-6-carboxylic acid hydrochloride
Sinónimos:
PPA directing group
About This Item
Productos recomendados
form
powder or crystals
Quality Level
reaction suitability
reaction type: C-C Bond Formation
reagent type: catalyst
reagent type: ligand
reaction type: C-H Activation
InChI
1S/C11H8N2O2/c14-11(15)10-6-3-5-9(13-10)8-4-1-2-7-12-8/h1-7H,(H,14,15)
InChI key
ZQTILGDVDYWICD-UHFFFAOYSA-N
Categorías relacionadas
Application
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
Lo sentimos, en este momento no disponemos de COAs para este producto en línea.
Si necesita más asistencia, póngase en contacto con Atención al cliente
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Contenido relacionado
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
The Engle lab strives to invent novel catalytic alkene and alkyne functionalization methods to expedite organic synthesis. These transformations offer a powerful platform for conversion of simple, abundant, and planar starting materials into densely functionalized, stereochemically complex products in a single step. To this end, the Engle lab has developed various substrate directivity strategies in which native functional groups can be temporarily masked with auxiliaries that are capable of reversibly binding the metal catalyst, thereby enhancing kinetic reactivity, suppressing unwanted side reactions, and facilitating high selectivity. The Engle lab works with us to make synthetically enabling directing groups, catalysts, and ligands readily available to the synthetic community for reaction discovery and small-molecule synthesis.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico