796549
Stahl Aerobic Oxidation TEMPO solution
0.2 M in acetonitrile, Solution for Oxidation of Primary Alcohols
About This Item
Productos recomendados
Quality Level
form
liquid
reaction suitability
reagent type: oxidant
concentration
0.2 M in acetonitrile
storage temp.
2-8°C
SMILES string
CN1C=CN=C1.CC2(C)CCCC(C)(C)N2[O].C3(C4=NC=CC=C4)=NC=CC=C3
InChI
1S/C10H8N2.C9H18NO.C4H6N2/c1-3-7-11-9(5-1)10-6-2-4-8-12-10;1-8(2)6-5-7-9(3,4)10(8)11;1-6-3-2-5-4-6/h1-8H;5-7H2,1-4H3;2-4H,1H3
InChI key
BQFURWVGIDXRNB-UHFFFAOYSA-N
General description
Application
related product
signalword
Danger
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1C
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
35.6 °F
flash_point_c
2.0 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Alcohol oxidation yields aldehydes and ketones, crucial intermediates in organic synthesis.
Contenido relacionado
he Stahl Lab focuses on the development of catalysts and catalytic reactions for selective oxidation of organic molecules, with particular emphasis on aerobic oxidation reactions.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
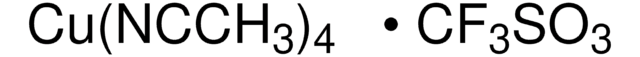
![9-Azabicyclo[3.3.1]nonane N-oxyl 95%](/deepweb/assets/sigmaaldrich/product/structures/287/155/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf/640/e2f4a2e1-1d4e-4bed-9187-9e16d23cbbbf.png)



![1,8-Diazabiciclo[5.4.0]undec-7-eno 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)









