771465
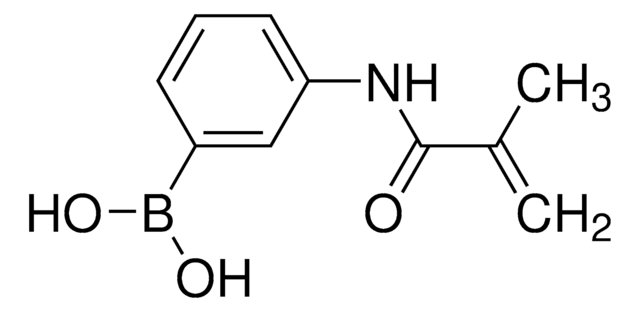
3-(Acrylamido)phenylboronic acid
98%
Sinónimos:
3-(Propenamido)phenylboronic acid, N-Acryloyl-3-aminophenylboronic acid, Boronic acid acrylamide, Phenylboronate acrylamide
About This Item
Productos recomendados
Quality Level
assay
98%
form
powder
mp
129-146 °C
storage temp.
2-8°C
SMILES string
OB(O)c1cccc(NC(=O)C=C)c1
InChI
1S/C9H10BNO3/c1-2-9(12)11-8-5-3-4-7(6-8)10(13)14/h2-6,13-14H,1H2,(H,11,12)
InChI key
ULVXDHIJOKEBMW-UHFFFAOYSA-N
General description
Application
- As a monomer to synthesize poly(methacrylic acid)-co-3-(acrylamido)phenylboronic acid (PMAA-co-AAPBA) copolymer as a supramolecular receptor for biosensor applications. AAPBA helps to enhance the water solubility and binding affinity of the copolymer. This copolymer is utilized for carbohydrate sensing in an aqueous medium.
- As a monomer to prepare poly(3-Acrylamidophenyl boronic acid-b-diethylene glycol dimethacrylate) for the fabrication of glucose-sensitive nanoparticles for insulin delivery. The specific interactions of AAPBA with the diol moiety present in glucose molecules induce glucose responsiveness into the block copolymer.
- As a monomer and cross-linker to synthesize self-healing composite hydrogels for tissue engineering and drug delivery systems. They can mimic the properties of natural tissues and provide a suitable environment for cell growth. AAPBA polymerizes with acrylamide and simultaneously interacts with cis-diol of hydroxypropyl guar gum (HPG) to facilitate the formation of hydrogel with good mechanical strength and fast self-healing properties.
signalword
Warning
hcodes
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico